Ranatensin-HL: A Bombesin-Related Tridecapeptide from the Skin Secretion of the Broad-Folded Frog, Hylarana latouchii
Abstract
:1. Introduction
2. Results
2.1. Identification and Structural Characterisation of Ranatensin-HL
2.2. Molecular Cloning of Ranatensin-HL Biosynthetic Precursor-Encoding cDNA
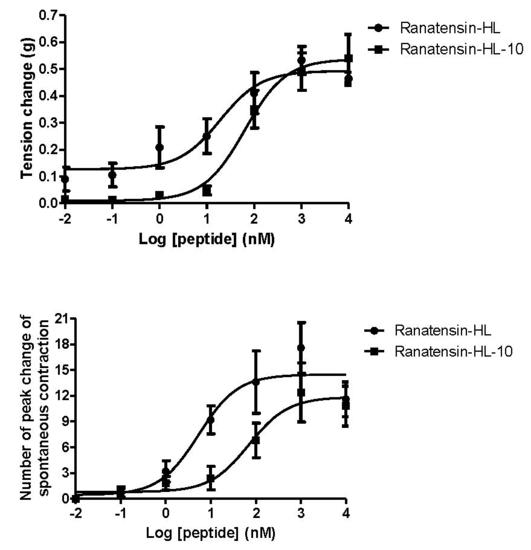
2.3. Smooth Muscle Pharmacology
3. Discussion
4. Materials and Methods
4.1. Specimen Biodata and Secretion Harvesting
4.2. Reverse-Phase HPLC Fractionation of Skin Secretion
4.3. Bioactivity Screening Using Rat Urinary Bladder and Uterus Smooth Muscles
4.4. Identification and Structural Characterisation of Peptide Possessing Bioactivity
4.5. Molecular Cloning of the Peptide Biosynthetic Precursor-Encoding cDNA
4.6. Solid-Phase Peptide Synthesis of Ranatensin-HL and Ranatensin-HL-10
4.7. Pharmacological Assay of Synthetic Peptides Using the Rat Urinary Bladder and Uterus Smooth Muscles
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| HPLC | High-Performance Liquid Chromatography |
| NCBI-BLASTp | National Centre for Biotechnology Information-Basic Local Alignment Search Tool Protein Database |
| NCBI-BLASTn | National Centre for Biotechnology Information-Basic Local Alignment Search Tool Nucleotide Database |
| RACE | Rapid Amplification of cDNA Ends |
| ORF | Open-Reading Frame |
| EMBL | European Molecular Biology Laboratory |
| Fmoc | 9-Fluorenylmethyloxycarbonyl |
| TFA | Trifluoroacetic Acid |
| IACUC | Institutional Animal Care and Use Committee |
| MALDI-TOF MS | Matrix-Assisted Laser Desorption/Ionization Time-of-Flight Mass Spectrometry |
| NUP | Nested Universal Primer |
References
- König, E.; Binindaemonds, O.R.; Shaw, C. The diversity and evolution of anuran skin peptides. Peptides 2015, 63, 96–117. [Google Scholar] [CrossRef] [PubMed]
- Rajchard, J. Sex pheromones in amphibians: A review. Vet. Med. 2005, 50, 383–389. [Google Scholar]
- Conlon, J.M.; Leprince, J. Identification and analysis of bioactive peptides in amphibian skin secretions. Methods Mol. Biol. 2010, 615, 145–157. [Google Scholar] [PubMed]
- Anastasi, A.; Erspamer, V.; Bucci, M. Isolation and structure of bombesin and alytesin, two analogous active peptides from the skin of the European amphibians Bombina and Alytes. Experientia 1971, 27, 166–167. [Google Scholar] [CrossRef] [PubMed]
- Yasuhara, T.; Nakajima, T.; Nokihara, K.; Yanaihara, C.; Yanaihara, N.; Erspamer, V.; Erspamer, G.F. Two new frog skin peptides, phyllolitorins of the bombesin-ranatensin family from Phyllomedusa sauvagei. Biomed. Res. 1983, 4, 407–412. [Google Scholar]
- Nakajima, T.; Tanimura, T.; Pisano, J. Isolation and structure of a new vasoactive polypeptide. Fed. Proc. 1970, 29, 282. [Google Scholar]
- Xu, X.; Lai, R. The chemistry and biological activities of peptides from amphibian skin secretions. Chem. Rev. 2015, 115, 1760–1846. [Google Scholar] [CrossRef] [PubMed]
- McDonald, T.; Jӧrnvall, H.; Nilsson, G.; Vagne, M.; Ghatei, M.; Bloom, S.; Mutt, V. Characterization of a gastrin releasing peptide from porcine non-antral gastric tissue. Biochem. Biophys. Res. Commun. 1979, 90, 227–233. [Google Scholar] [CrossRef]
- Minamino, N.; Kangawa, K.; Matsuo, H. Neuromedin B: A novel bombesin-like peptide identified in porcine spinal cord. Biochem. Biophys. Res. Commun. 1983, 114, 541–548. [Google Scholar] [CrossRef]
- Ramos-Álvarez, I.; Moreno, P.; Mantey, S.A.; Nakamura, T.; Nuche-Berenguer, B.; Moody, T.W.; Coy, D.H.; Jensen, R.T. Insights into bombesin receptors and ligands: Highlighting recent advances. Peptides 2015, 72, 128–144. [Google Scholar] [CrossRef] [PubMed]
- Lai, R.; Liu, H.; Lee, W.H.; Zhang, Y. A novel proline rich bombesin-related peptide (PR-bombesin) from toad Bombina maxima. Peptides 2002, 23, 437–442. [Google Scholar] [CrossRef]
- Nagalla, S.R.; Barry, B.J.; Falick, A.M.; Gibson, B.W.; Taylor, J.E.; Dong, J.Z.; Spindel, E.R. There are three distinct forms of bombesin: Identification of [Leu13] bombesin, [Phe13] bombesin, and [Ser3, Arg10, Phe13] bombesin in the frog Bombina Orientalis. J. Biol. Chem. 1996, 271, 7731–7737. [Google Scholar] [CrossRef] [PubMed]
- Krane, I.; Naylor, S.; Helin-Davis, D.; Chin, W.W.; Spindel, E. Molecular cloning of cDNAs encoding the human bombesin-like peptide neuromedin B. Chromosomal localization and comparison to cDNAs encoding its amphibian homolog ranatensin. J. Biol. Chem. 1988, 263, 13317–13323. [Google Scholar] [PubMed]
- Jensen, R.; Battey, J.; Spindel, E.; Benya, R. International Union of Pharmacology. LXVIII. Mammalian bombesin receptors: Nomenclature, distribution, pharmacology, signaling, and functions in normal and disease states. Pharmacol. Rev. 2008, 60, 1–42. [Google Scholar] [CrossRef] [PubMed]
- Spindel, E.R.; Gibson, B.W.; Reeve, J.R.; Kelly, M. Cloning of cDNAs encoding amphibian bombesin: Evidence for the relationship between bombesin and gastrin-releasing peptide. Proc. Natl. Acad. Sci. USA 1990, 87, 9813–9817. [Google Scholar] [CrossRef] [PubMed]
- Ohki-Hamazaki, H. Neuromedin B. Prog. Neurobiol. 2000, 62, 297–312. [Google Scholar] [CrossRef]
- Gonzalez, N.; Moody, T.W.; Igarashi, H.; Ito, T.; Jensen, R.T. Bombesin-related peptides and their receptors: Recent advances in their role in physiology and disease states. Curr. Opin. Endocrinol. Diabetes Obes. 2008, 15, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Ohki-Hamazaki, H.; Iwabuchi, M.; Maekawa, F. Development and function of bombesin-like peptides and their receptors. Int. J. Dev. Biol. 2005, 49, 293–300. [Google Scholar] [CrossRef] [PubMed]
- Engster, K.M.; Kroczek, A.L.; Rose, M.; Stengel, A.; Kobelt, P. Peripheral injection of bombesin induces c-Fos in NUCB2/nesfatin-1 neurons. Brain Res. 2016, 1648, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Saito, H.; Nakamachi, T.; Inoue, K.; Ikeda, R.; Kitamura, K.; Minamino, N.; Shioda, S.; Miyata, A. Autocrine effects of neuromedin B stimulate the proliferation of rat primary osteoblasts. J. Endocrinol. 2013, 217, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Pierre, J.F.; Neuman, J.C.; Brill, A.L.; Brar, H.K.; Thompson, M.F.; Cadena, M.T.; Connors, K.M.; Busch, R.A.; Heneghan, A.F.; Cham, C.M.; et al. The gastrin-releasing peptide analog bombesin preserves exocrine and endocrine pancreas morphology and function during parenteral nutrition. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G431–G442. [Google Scholar] [CrossRef] [PubMed]
- Wechselberger, C.; Kreil, G.; Richter, K. Isolation and sequence of a cDNA encoding the precursor of a bombesin like peptide from brain and early embryos of Xenopus laevis. Proc. Natl. Acad. Sci. USA 1992, 89, 9819–9822. [Google Scholar] [CrossRef] [PubMed]
- Simmaco, M.; Mignogna, G.; Barra, D.; Bossa, F. Antimicrobial peptides from skin secretions of Rana esculenta—Molecular cloning of cDNAs encoding and isolation of new active peptides. J. Biol. Chem. 1994, 269, 11956–11961. [Google Scholar] [PubMed]
- Lin, Y.; Hu, N.; Lyu, P.; Ma, J.; Wang, L.; Zhou, M.; Guo, S.; Chen, T.; Shaw, C. Hylaranins: Prototypes of a new class of amphibian antimicrobial peptide from the skin secretion of the oriental broad-folded frog, Hylarana latouchii. Amino Acids 2014, 46, 901–909. [Google Scholar] [CrossRef] [PubMed]
- Erspamer, V. Bioactive secretions of the amphibian integument. Amphibian Biol. 1994, 1, 178–350. [Google Scholar]
- Clarke, B.T. The natural history of amphibian skin secretions, their normal functioning and potential medical applications. Biol. Rev. Camb. Philos. Soc. 1997, 72, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Bevins, C.L.; Zasloff, M. Peptides from frog skin. Annu. Rev. Biochem. 1990, 59, 395–414. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Li, W.; Duan, L.; Xiao, Y. A bombesin-like peptide from skin of Sanguirana varians. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2010, 155, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Bian, J.; Chen, Z.; Miao, Y.; Li, W. A novel bombesin-like peptide from skin of Rana shuchinae. Mol. Biol. Rep. 2011, 38, 3599–3603. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yu, H.; Xu, X.; Wang, X.; Liu, D.; Lai, R. Multiple bombesin-like peptides with opposite functions from skin of Odorrana grahami. Genomics 2007, 89, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Shaw, C. Identification and molecular cloning of novel trypsin inhibitor analogs from the dermal venom of the Oriental fire-bellied toad (Bombina orientalis) and the European yellow-bellied toad (Bombina variegata). Peptides 2003, 24, 873–880. [Google Scholar] [CrossRef]
- Chen, X.; He, W.; Lei, W.; Mei, Z.; Chen, T.; Shaw, C. Identification of miscellaneous peptides from the skin secretion of the European edible frog, Pelophylax kl. Esculentus. Protein J. 2016, 35, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Tyler, M.J.; Stone, D.; Bowie, J.H. A novel method for the release and collection of dermal, glandular secretions from the skin of frogs. J. Pharmacol. Toxicol. Methods 1992, 28, 199–200. [Google Scholar] [CrossRef]
Sample Availability: Samples of the ranatensin-HL and ranatensin-HL-10 are available from the authors. |








© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.; Chen, T.; Zhou, M.; Wang, L.; Su, S.; Shaw, C. Ranatensin-HL: A Bombesin-Related Tridecapeptide from the Skin Secretion of the Broad-Folded Frog, Hylarana latouchii. Molecules 2017, 22, 1110. https://doi.org/10.3390/molecules22071110
Lin Y, Chen T, Zhou M, Wang L, Su S, Shaw C. Ranatensin-HL: A Bombesin-Related Tridecapeptide from the Skin Secretion of the Broad-Folded Frog, Hylarana latouchii. Molecules. 2017; 22(7):1110. https://doi.org/10.3390/molecules22071110
Chicago/Turabian StyleLin, Yan, Tianbao Chen, Mei Zhou, Lei Wang, Songkun Su, and Chris Shaw. 2017. "Ranatensin-HL: A Bombesin-Related Tridecapeptide from the Skin Secretion of the Broad-Folded Frog, Hylarana latouchii" Molecules 22, no. 7: 1110. https://doi.org/10.3390/molecules22071110
APA StyleLin, Y., Chen, T., Zhou, M., Wang, L., Su, S., & Shaw, C. (2017). Ranatensin-HL: A Bombesin-Related Tridecapeptide from the Skin Secretion of the Broad-Folded Frog, Hylarana latouchii. Molecules, 22(7), 1110. https://doi.org/10.3390/molecules22071110