Synthesis of Compounds of the Pyrimidine Series Based on the Reactions of 3-Arylmethylidenefuran-2(3H)-ones with N,N-Binucleophilic Reagents
Abstract
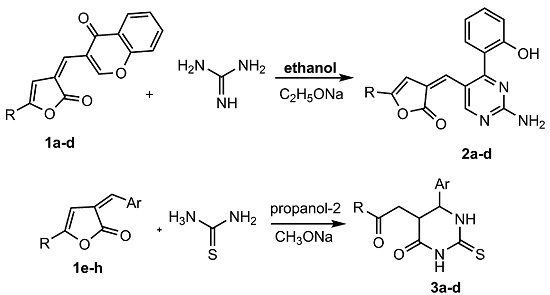
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Synthesis of Compounds 2a–d
3.3. Synthesis of Compounds 3a–d
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kim, Y.H.; Lee, N.J. Synthesis of 2-amino-dihydro-4(1H)-pyrimidininone derivatives: Cyclization of N-substituted guanidines with α,β-unsaturated ketones. Heterocycles 1983, 20, 1769–1772. [Google Scholar] [CrossRef]
- Madhavan, G.R.; Chakrabarti, R.; Vikramadithyan, R.K.; Mamidi, R.N.V.S.; Balraju, V.; Rajesh, B.M.; Misra, P.; Kumar, S.K.B.; Lohray, B.B.; Lohray, V.B.; et al. Synthesis and biological activity of novel pirimidininone containing thiazolidinedione derivatives. Bioorg. Med. Chem. 2002, 10, 2671–2680. [Google Scholar] [CrossRef]
- Gangiee, A.; Adaira, O.; Queener, S.F. Synthesis of 2,4-diamino-6-(thioarylmethyl)pyrido[2,3-d]pyrimidines as dihydrofolate reductase inhiditors. Bioorg. Med. Chem. 2001, 9, 2929–2935. [Google Scholar] [CrossRef]
- Saladino, R.; Ciambecchini, U.; Maga, G.; Mastromarino, P.; Conti, C.; Botta, M. A new and efficient synthesis of substituted 6-[(2’-dialkylamino)ethyl]pyrimidine and 4-N,N-dialkyl-6-vinyl-cytosine derivative and evaluation of their anti-rubella activity. Bioorg. Med. Chem. 2002, 10, 2143–2153. [Google Scholar] [CrossRef]
- Skulnick, H.I.; Weed, S.D.; Eidson, E.E.; Renis, H.E.; Stringfellow, D.A.; Wierenga, W. Pyrimidinones. 1. 2-amino-5-halo-6-aryl-4(3H)-pirimidinones. Interferon-Inducing antiviral agents. J. Med. Chem. 1985, 28, 1864–1869. [Google Scholar] [CrossRef] [PubMed]
- Dave, C.D.; Shah, R.D. Annellation of triazole and tetrazole systems onto pyrrolo[2,3-d]pyrimidines: Synthesis of tetrazolo[1,5-c]-pyrrolo[3,2-e]pyrimidines and triazolo[1,5-c]pyrrolo-[3,2-e]pyrimidines as potential antibacterial agents. Molecules 2002, 7, 554–565. [Google Scholar] [CrossRef]
- Zuniga, E.S.; Korkegian, A.; Mullen, S.; Hembre, E.J.; Ornstein, P.L.; Cortez, G.; Biswas, K.; Naresh, K.; Cramer, J.; Masquelin, T.; et al. The synthesis and evaluation of triazolopyrimidines as anti-tubercular agents. Bioorg. Med. Chem. 2017, 25, 3922–3946. [Google Scholar] [CrossRef] [PubMed]
- Pretorius, S.I.; Breytenbach, W.J.; de Kock, C.; Smith, P.J.; N’Da, D.D. Synthesis, characterization and antimalarial activity of quinolone-pyrimidine hybrids. Bioorg. Med. Chem. 2013, 21, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Brogden, R.N.; Carmine, A.A.; Heel, R.C.; Speight, T.M.; Avery, G.S. Trimethoprim: A review of its antibacterial activity, pharmacokinetics and therapeutic use in urinary tract infections. Drugs 1982, 23, 405–430. [Google Scholar] [CrossRef] [PubMed]
- Millan, M.J.; Cussac, D.; Milligan, G.; Carr, C.; Audinot, V.; Gobert, A.; Lejeune, F.; Rivet, J.M.; Brocco, M.; Duqueyroix, D.; et al. Antiparkinsonian agent piribedil displays antagonist properties at native, rat, and cloned, human α2-adrenoceptors: Cellular and functional characterization. J. Pharmacol. Exp. Ther. 2001, 297, 876–887. [Google Scholar] [PubMed]
- De Clercq, E.; Field, H.J. Antiviral prodrugs—The development of successful prodrug strategies for antiviral chemotherapy. Br. J. Pharmacol. 2006, 147, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Türkoğlu, E.A.; Şentürk, M.; Supuran, C.T.; Ekinci, D. Carbonic anhydrase inhibitory properties of some uracil derivatives. J. Enzyme Inhib. Med. Chem. 2017, 32, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Squarcialupi, L.; Betti, M.; Catarzi, D.; Varano, F.; Falsini, M.; Ravani, A.; Pasquini, S.; Vincenzi, F.; Salmaso, V.; Sturlese, M.; et al. The role of 5-arylalkylamino- and 5-piperazino- moieties on the 7-aminopyrazolo[4,3-d]pyrimidine core in affecting adenosine A1 and A2A receptor affinity and selectivity profiles. J. Enzyme Inhib. Med. Chem. 2017, 32, 248–263. [Google Scholar] [CrossRef] [PubMed]
- Naguib, B.H.; El-Nassan, H.B.; Abdelghany, T.M. Synthesis of new pyridothienopyrimidinone derivatives as Pim-1 inhibitors. J. Enzyme Inhib. Med. Chem. 2017, 32, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Kamneva, I.E.; Verevochkin, A.A.; Zheleznova, M.A.; Yegorova, A.Y. Reactions of 3-arylmethylene-3H-furan(pyrrol)-2-ones with azomethine ylide: Synthesis of substituted azaspirononenes. Heterocycl. Commun. 2016, 22, 255–257. [Google Scholar] [CrossRef]
- Anis’kova, T.; Kamneva, I.; Egorova, A. The reactions of arylmethylidene(ethylidene)furanones with N-nucleophiles. Lett. Org. Chem. 2016, 13, 699–705. [Google Scholar] [CrossRef]
- Anis’kova, T.V.; Yegorova, A.Y.; Chadina, V.V. Reactions of 5-aryl-3-arylmethylene substituted 3H-furan-2-ones and 3H-pyrrol-2-ones with acetoacetic ester. Mendeleev Commun. 2008, 18, 167–168. [Google Scholar] [CrossRef]
- Anis’kova, T.V.; Chadina, V.V.; Yegorova, A.Y. Reaction of 3-arylmethylidene-3H-furan-2-ones with 3-amino-1,2,4-triazole as a convenient technique to synthesize condensed diazepinones. Synth. Commun. 2011, 41, 2315–2322. [Google Scholar] [CrossRef]
- Anis’kova, T.V.; Kamneva, I.E.; Egorova, A.Y. Synthesis of arylmethylidene(ethylidene)furanones. Rev. J. Chem. 2014, 4, 204–220. [Google Scholar] [CrossRef]
- Anis’kova, T.V.; Yegorova, A.Y.; Chadina, V.V. Synthesis of polyheterocyclic compounds derived from 6-amino-4-aryl-2-r-4H-furo[2,3-b]pyran-5-carbonitriles. Chem. Heterocycl. Compd. 2009, 45, 1460–1463. [Google Scholar] [CrossRef]
- Anis’kova, T.V.; Egorova, A.Y. Synthesis of new furopyrans and angularly fused furopyranochromenes from 3-arylmethylidenefuran-2-ones. Russ. J. Org. Chem. 2013, 49, 1514–1516. [Google Scholar] [CrossRef]
- Anis’kova, T.V.; Stulova, E.G.; Egorova, A.Y. Reaction of 5-arylfuran-2(3H)-ones with 3-formylchromone. Russ. J. Org. Chem. 2016, 52, 1215–1216. [Google Scholar] [CrossRef]
- Anis’kova, T.V.; Yegorova, A.Y. Synthesis of angular trioxacyclopentaphenanthrenes by the reaction of substituted 2-furanones with malonic acid dinitrile. Chem. Heterocycl. Compd. 2011, 47, 514–516. [Google Scholar] [CrossRef]
- Anis’kov, A.A.; Kamneva, I.Y.; Yegorova, A.Y.; Zheleznova, M.A. Reaction of arylmethylidene derivatives of 3H-furan-2-ones with azomethine ylide. Chem. Heterocycl. Compd. 2015, 51, 709–712. [Google Scholar] [CrossRef]
- Anis’kova, T.V.; Yegorova, A.Y. Synthesis of 6-amino-4-aryl-2R-4H-furo[2,3-b]pyran-5-carbo- nitriles based on the condensation of 3-arylmethylene-3H-furan-2-ones with malononitrile. Chem. Heterocycl. Compd. 2009, 45, 662–665. [Google Scholar] [CrossRef]
- Anis’kova, T.V.; Verevochkin, A.A.; Yegorova, A.Y. Synthesis of Substituted 3,4-Dihydrofuro[2,3-d]pyrimidines from 3-arylmethylidenefuran-2(3H)-ones. Russ. J. Org. Chem. 2016, 52, 1861–1862. [Google Scholar] [CrossRef]
- Grinev, V.S.; Burukhina, O.V.; Gosenova, O.L.; Apanasova, N.V.; Egorova, A.Y. The influence of new plant-growth regulators of benzimidazole and tyazine series on corn (Zea Mays L.). Agrokhimiya (Agrochemistry) 2013, 7, 42–48. [Google Scholar]
- Sedavkina, V.A.; Morozova, N.A.; Egorova, A.Y.; Ostroumov, I.G. Synthesis of 5-alkyl-3H-thiolen-2-ones and 5-alkyl-3H-furan-2-ones and condensation reactions at the heterocyclic methylene group. Chem. Heterocycl. Compd. 1987, 23, 377–380. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 2a–d, 3a–d are available from the authors. |




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aniskova, T.; Grinev, V.; Yegorova, A. Synthesis of Compounds of the Pyrimidine Series Based on the Reactions of 3-Arylmethylidenefuran-2(3H)-ones with N,N-Binucleophilic Reagents. Molecules 2017, 22, 1251. https://doi.org/10.3390/molecules22081251
Aniskova T, Grinev V, Yegorova A. Synthesis of Compounds of the Pyrimidine Series Based on the Reactions of 3-Arylmethylidenefuran-2(3H)-ones with N,N-Binucleophilic Reagents. Molecules. 2017; 22(8):1251. https://doi.org/10.3390/molecules22081251
Chicago/Turabian StyleAniskova, Tatyana, Vyacheslav Grinev, and Alevtina Yegorova. 2017. "Synthesis of Compounds of the Pyrimidine Series Based on the Reactions of 3-Arylmethylidenefuran-2(3H)-ones with N,N-Binucleophilic Reagents" Molecules 22, no. 8: 1251. https://doi.org/10.3390/molecules22081251
APA StyleAniskova, T., Grinev, V., & Yegorova, A. (2017). Synthesis of Compounds of the Pyrimidine Series Based on the Reactions of 3-Arylmethylidenefuran-2(3H)-ones with N,N-Binucleophilic Reagents. Molecules, 22(8), 1251. https://doi.org/10.3390/molecules22081251