Covalent Porphyrin Hybrids Linked with Dipyrrin, Bidipyrrin or Thiacorrole
Abstract
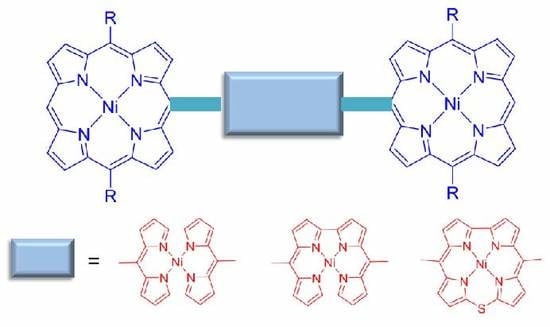
:1. Introduction
2. Results and Discussion
3. Materials and Synthesis
3.1. Materials and Instrumentation
3.2. Crystallography
3.3. Syntheses
3.3.1. Synthesis of 2
3.3.2. Synthesis of 3
3.3.3. Synthesis of 4
3.3.4. Synthesis of 5
3.3.5. Synthesis of 6
3.3.6. Synthesis of 7
3.3.7. Synthesis of 8
4. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Maruccio, G.; Cingolani, R.; Rinaldi, R. Projecting the nanoworld: Concepts, results and perspectives of molecular electronics. J. Mater. Chem. 2004, 14, 542–554. [Google Scholar] [CrossRef]
- Fabian, J.; Nakazumi, H.; Matsuoka, M. Near-infrared absorbing dyes. Chem. Rev. 1992, 92, 1197–1226. [Google Scholar] [CrossRef]
- Nalwa, H.S. Organic Materials for Third-Order Nonlinear Optics. Adv. Mater. 1993, 5, 341–358. [Google Scholar] [CrossRef]
- Marrocchi, A.; Facchetti, A.; Lanari, D.; Petrucci, C.; Vaccaro, L. Current methodologies for a sustainable approach to π-conjugated organic semiconductors. Energy Environ. Sci. 2016, 9, 763–786. [Google Scholar] [CrossRef]
- Ding, Y.B.; Tang, Y.Y.; Xie, Y.S. Fluorescent and colorimetric ion probes based on conjugated oligopyrroles. Chem. Soc. Rev. 2015, 44, 1101–1112. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.B.; Zhu, W.H.; Xie, Y.S. Development of Ion Chemosensors Based on Porphyrin Analogues. Chem. Rev. 2017, 177, 2203–2256. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.B.; Li, T.; Zhu, W.H. Highly selective colorimetric sensing of cyanide based on formation of dipyrrin adducts. Org. Biomol. Chem. 2012, 10, 4201–4207. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.B.; Xie, Y.S.; Li, X.; Hill, J.P.; Zhang, W.B.; Zhu, W.H. Selective and sensitive “turn-on” fluorescent Zn2+ sensors based on di-and tripyrrins with readily modulated emission wavelengths. Chem. Commun. 2011, 47, 5431–5433. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.G.; Xie, Y.S.; Ding, Y.B.; Zhu, W.H. Colorimetric fluoride sensors based on deprotonation of pyrrole–hemiquinone compounds. Chem. Commun. 2010, 46, 3669–3671. [Google Scholar] [CrossRef] [PubMed]
- Saito, S.; Osuka, A. Expanded porphyrins: Intriguing structures, electronic properties, and reactivities. Angew. Chem. Int. Ed. 2011, 50, 4342–4373. [Google Scholar] [CrossRef] [PubMed]
- Stepien, M.; Sprutta, N.; Latos-Grazynski, L. Figure eights, Möbius bands, and more: Conformation and aromaticity of porphyrinoids. Angew. Chem. Int. Ed. 2011, 50, 4288–4340. [Google Scholar] [CrossRef] [PubMed]
- Roznyatovskiy, V.V.; Lee, C.H.; Sessler, J.L. π-Extended isomeric and expanded porphyrins. Chem. Soc. Rev. 2013, 42, 1921–1933. [Google Scholar] [CrossRef] [PubMed]
- Gill, H.S.; Finger, I.; Božidarević, S.F.; Scott, M.J. Preparation of α, β-unsubstituted meso-arylbidipyrrins via metal-templated, oxidative coupling of dipyrrins. New J. Chem. 2005, 29, 68–71. [Google Scholar] [CrossRef]
- Hashimoto, T.; Nishimura, T.; Lim, J.M.; Kim, D.; Maeda, H. Formation of Metal-Assisted Stable Double Helices in Dimers of Cyclic Bis-Tetrapyrroles that Exhibit Spring-Like Motion. Chem. Eur. J. 2010, 16, 11653–11661. [Google Scholar] [CrossRef] [PubMed]
- Ruffin, H.; Baudron, A.S.; Salazar-Mendoza, D.; Hosseini, W. A Silver Bite: Crystalline Heterometallic Architectures Based on Ag–π Interactions with a Bis-Dipyrrin Zinc Helicate. Chem. Eur. J. 2014, 20, 2449–2453. [Google Scholar] [CrossRef] [PubMed]
- Baudron, S.A.; Ruffn, H.; Hosseini, M.W. On Zn(ii) 2,2′-bisdipyrrin circular helicates. Chem. Commun. 2015, 51, 5906–5909. [Google Scholar] [CrossRef] [PubMed]
- Lakshmi, V.; Lee, W.-Z.; Ravikanth, M. Synthesis, structure and spectral and electrochemical properties of 3-pyrrolyl BODIPY-metal dipyrrin complexes. Dalton Trans. 2014, 43, 16006–16014. [Google Scholar] [CrossRef] [PubMed]
- Sakamoto, R.; Iwashima, T.; Tsuchiya, M.; Toyoda, R.; Matsuoka, R.; Kögel, J.F.; Kusaka, S.; Hoshiko, K.; Yagi, T.; Nagayama, T.; et al. New aspects in bis and tris(dipyrrinato) metal complexes: Bright luminescence, self-assembled nanoarchitectures, and materials applications. J. Mater. Chem. A 2015, 3, 15357–15371. [Google Scholar] [CrossRef]
- Gadekar, S.C.; Reddy, B.K.; Panchal, S.P.; Anand, V.G. Metal assisted cyclomerization of benzodipyrrins into expanded norroles, aza-heptalene and acyclic dimmers. Chem. Commun. 2016, 52, 4565–4568. [Google Scholar] [CrossRef] [PubMed]
- Lewtak, J.P.; Gryko, D.T. Synthesis of π-extended porphyrins via intramolecular oxidative coupling. Chem. Commun. 2012, 48, 10069–10086. [Google Scholar] [CrossRef] [PubMed]
- Senge, M.O.; Fazekas, M.E.; Notaras, G.A.; Blau, W.J.; Zawadzka, M.; Locos, O.B.; Ni Mhuircheartaigh, E.M. Nonlinear optical properties of porphyrins. Adv. Mater. 2007, 19, 2737–2774. [Google Scholar] [CrossRef]
- Pawlicki, M.; Collins, H.A.; Denning, R.G.; Anderson, H.L. Two-Photon Absorption and the Design of Two-Photon Dyes. Angew. Chem. Int. Ed. 2009, 48, 3244–3266. [Google Scholar] [CrossRef] [PubMed]
- Davis, N.K.S.; Thompson, A.L.; Anderson, H.L. A Porphyrin Fused to Four Anthracenes. J. Am. Chem. Soc. 2011, 133, 30–31. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.; Wilson, C.J.; Odell, B.; Anderson, P. Template-Directed Synthesis of a π-Conjugated Porphyrin Nanoring. Angew. Chem. Int. Ed. 2007, 46, 3122–3125. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Jang, S.Y.; Tanaka, T.; Aratani, N.; Lim, J.M.; Kim, K.S.; Kim, D.; Osuka, A. Two-Dimensionally Extended Porphyrin Tapes: Synthesis and Shape-Dependent Two-Photon Absorption Properties. Chem. Eur. J. 2008, 14, 8279–8289. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Aratani, N.; Shinokubo, H.; Takagi, A.; Kawai, T.; Matsumoto, T.; Yoon, Z.S.; Kim, D.Y.; Ahn, T.K.; Kim, D.; et al. A directly fused tetrameric porphyrin sheet and its anomalous electronic properties that arise from the planar cyclooctatetraene core. J. Am. Chem. Soc. 2006, 128, 4119–4127. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Lee, B.S.; Aratani, N.; Yoon, M.-C.; Kim, D.; Osukaet, A. Synthesis and properties of hybrid porphyrin tapes. Chem. Eur. J. 2011, 17, 14400–14412. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Tanaka, T.; Lee, B.S.; Kim, P.; Kim, D.; Osuka, A. An Electron-Deficient Porphyrin Tape. Chem. Asian J. 2012, 7, 1811–1816. [Google Scholar] [CrossRef] [PubMed]
- Bonifazi, D.; Scholl, M.; Song, F.Y.; Echegoyen, L.E.; Accorsi, G.; Armaroli, N.; Diederich, F. Exceptional redox and photophysical properties of a triply fused diporphyrin–C60 conjugate: Novel scaffolds for multicharge storage in molecular scale electronics. Angew. Chem. Int. Ed. 2003, 115, 5116–5120. [Google Scholar] [CrossRef]
- Kim, D.; Osuka, A. Directly linked porphyrin arrays with tunable excitonic interactions. Acc. Chem. Res. 2004, 37, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Aratani, N.; Kim, D.; Osuka, A. π-Conjugation Enlargement toward the Creation of Multi-Porphyrinic Systems with Large Two-Photon Absorption Properties. Chem. Asian J. 2009, 4, 1172–1182. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Tanaka, T.; Osuka, A. Fused porphyrinoids as promising near-infrared absorbing dyes. J. Mater. Chem. C 2013, 1, 2500–2519. [Google Scholar] [CrossRef]
- Tanaka, T.; Osuka, A. Conjugated porphyrin arrays: Synthesis, properties and applications for functional materials. Chem. Soc. Rev. 2015, 44, 943–969. [Google Scholar] [CrossRef] [PubMed]
- Davis, N.K.S.; Thompson, A.L.; Anderson, H.L. Bis-anthracene fused porphyrins: Synthesis, crystal structure, and near-IR absorption. Org. Lett. 2010, 12, 2124–2127. [Google Scholar] [CrossRef] [PubMed]
- Lindsey, J.S.; Prathapan, S.; Johnson, T.E.; Wagner, R.W. Porphyrin building blocks for modular construction of bioorganic model systems. Tetrahedron 1994, 50, 8941–8968. [Google Scholar] [CrossRef]
- Maruyama, K.; Kawabata, S. Synthesis and characterization of polyyne porphyrins. Bull. Chem. Soc. Jpn. 1990, 63, 170–175. [Google Scholar] [CrossRef]
- Tsuda, A.; Osuka, A. Fully conjugated porphyrin tapes with electronic absorption bands that reach into infrared. Science 2001, 293, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zhu, Y.-Z.; Fan, Q.-J.; Song, H.-B.; Zheng, J.-Y. Crystal Structure, and Spectroscopic Properties of Meso–Meso-Linked Porphyrin–Corrole Hybrids. Chem. Lett. 2013, 42, 936–938. [Google Scholar] [CrossRef]
- Murugavel, M.; Reddy, R.; Sankar, J. A new meso–meso directly-linked corrole–porphyrin–corrole hybrid: Synthesis and photophysical properties. RSC Adv. 2014, 4, 13669–13672. [Google Scholar] [CrossRef]
- Mori, H.; Tanaka, T.; Lee, S; Osuka, A.; Lim, J.M.; Kim, D.; Osuka, A. meso–meso Linked Porphyrin–[26]Hexaphyrin–Porphyrin Hybrid Arrays and Their Triply Linked Tapes Exhibiting Strong Absorption Bands in the NIR Region. J. Am. Chem. Soc. 2015, 137, 2097–2106. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Aratani, N.; Lim, J.M.; Osuka, A.; Kim, K.S.; Kim, D.; Osuka, A. Porphyrin–hexaphyrin hybrid tapes. Chem. Sci. 2011, 2, 1414–1418. [Google Scholar] [CrossRef]
- Li, F.R.; Yang, S.I.; Ciringh, Y.Z.; Seth, J.; Martin, C.H.; Singh, D.L.; Kim, D.; Birge, R.R.; Bocian, D.F.; Holten, D.; et al. Design, synthesis, and photodynamics of light-harvesting arrays comprised of a porphyrin and one, two, or eight boron-dipyrrin accessory pigments. J. Am. Chem. Soc. 1998, 120, 10001–10007. [Google Scholar] [CrossRef]
- Lee, C.Y.; Jang, J.K.; Kim, C.H.; Jung, J.; Park, B.K.; Park, J.; Choi, W.; Han, Y.K.; Joo, T.; Park, J.T.; et al. Remarkably efficient photocurrent generation based on a [60]fullerene–triosmium cluster/Zn–porphyrin/boron–dipyrrin triad SAM. Chem. Eur. J. 2010, 16, 5586–5599. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, H.; Kondo, T.; Sakida, T.; Yamaguchi, S.; Shinokubo, H. meso-Thiaporphyrinoids Revisited: Missing of Sulfur by Small Metals. Chem. Eur. J. 2012, 18, 16129–16135. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors |








| 3 | 4 |
|---|---|
| C114H114N12Ni3 | C114H112N12Ni3 |
| Mr = 1828.30 | Mr = 1826.29 |
| Orthorhombi | Triclinic |
| C222(1) | P-1 |
| a = 33.100(3) | a = 19.6380(18) |
| b = 24.600(2) | b = 20.1721(19) |
| c = 15.4400(14) | c = 27.330(3) |
| α = 90.00° | α = 73.257(2)° |
| β = 90.00° | β = 78.750(3)° |
| γ = 90.00° | γ = 67.5910(10)° |
| V = 12572.3(19) Å3 | V = 9540.1(15) Å3 |
| Z = 4 | Z = 3 |
| R1 = 0.1257 | R1 = 0.1264 |
| wR2 = 0.2693 | wR2 = 0.2897 |
| GOF = 1.038 | GOF = 1.084 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, R.; Yue, H.; Kong, J. Covalent Porphyrin Hybrids Linked with Dipyrrin, Bidipyrrin or Thiacorrole. Molecules 2017, 22, 1400. https://doi.org/10.3390/molecules22091400
He R, Yue H, Kong J. Covalent Porphyrin Hybrids Linked with Dipyrrin, Bidipyrrin or Thiacorrole. Molecules. 2017; 22(9):1400. https://doi.org/10.3390/molecules22091400
Chicago/Turabian StyleHe, Renbao, Huan Yue, and Jiahui Kong. 2017. "Covalent Porphyrin Hybrids Linked with Dipyrrin, Bidipyrrin or Thiacorrole" Molecules 22, no. 9: 1400. https://doi.org/10.3390/molecules22091400
APA StyleHe, R., Yue, H., & Kong, J. (2017). Covalent Porphyrin Hybrids Linked with Dipyrrin, Bidipyrrin or Thiacorrole. Molecules, 22(9), 1400. https://doi.org/10.3390/molecules22091400