GC-MS Analysis of the Volatile Constituents in the Leaves of 14 Compositae Plants
Abstract
:1. Introduction
2. Results
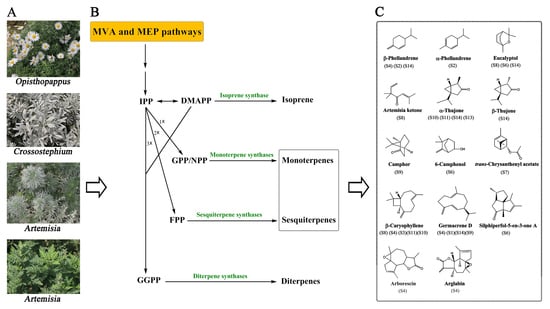
2.1. Identification and Quantification of the Chemical Constituents in 14 Compositae Plants
2.2. Volatile Composition Patterns in 14 Compositae Plants
2.3. PCA Analysis of 14 Compositae Plants Based on Terpenoid Compounds
2.4. HCA Analysis of 14 Compositae Plants Based on Terpenoid Compounds
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Sample Preparation
4.3. GC-MS Conditions
4.4. Peak Identification
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author contributions
Conflicts of Interest
References
- Dudareva, N.; Negre, F. Practical applications of research into the regulation of plant volatile emission. Curr. Opin. Plant Biol. 2005, 8, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Obistioiu, D.; Cristina, R.T.; Schmerold, I.; Chizzola, R.; Stolze, K.; Nichita, I.; Chiurciu, V. Chemical characterization by gc-ms and in vitro activity against Candida albicans of volatile fractions prepared from artemisia dracunculus, artemisia abrotanum, artemisia absinthium and artemisia vulgaris. Chem. Central J. 2014, 8, 6. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.H.; Zhang, S.F. A new variety of dendranthema gaertn. From shennongjia. J. Wuhan Bot. Res. 1983, 1, 237–238. [Google Scholar]
- Wang, C.Z.; Su, Y.; Li, D.; Cai, B.; Guo, Y.L. Analysis of volatile organic compounds from dendranthema indicum var Aromaticum by headspace gas chromatography-mass spectrometry and accurate mass measurement. Anal. Lett. 2010, 43, 2297–2310. [Google Scholar]
- Shih, C. Opisthopappus shih—A new genus of compositae from china. Acta Phytotaxon. Sin. 1979, 17, 110–112. [Google Scholar]
- Sun, H.; Zhang, F.; Chen, S.; Guan, Z.; Jiang, J.; Fang, W.; Chen, F. Effects of aphid herbivory on volatile organic compounds of artemisia annua and chrysanthemum morifolium. Biochem. Syst. Ecol. 2015, 60, 225–233. [Google Scholar] [CrossRef]
- Mucciarelli, M.; Caramiello, R.; Maffei, M.; Chialva, F. Essential oils from some Artemisia species growing spontaneously in north-west italy. Flavour Fragr. J. 1995, 10, 25–32. [Google Scholar] [CrossRef]
- Choi, J.Y.; Cho, I.H.; Kim, Y.-S.; Lee, H.J. Aroma-active compounds of korean mugwort (Artemisia princeps orientalis). J. Korean Soc. Appl. Biol. Chem. 2014, 57, 323–329. [Google Scholar] [CrossRef]
- Lin, Y.R. The nomenclature of the medicinal Artemisia in early chinese pharmacopoeias. Bull. Bot. Res. 1991, 11, 1–24. [Google Scholar]
- Chagonda, L.S.; Makanda, C.; Chalchat, J.C. The essential oil of cultivated Artemisia afra (jacq.) from zimbabwe. Flavour Fragr. J. 1999, 14, 140–142. [Google Scholar] [CrossRef]
- Bourgou, S.; Tammar, S.; Salem, N.; Mkadmini, K.; Msaada, K. Phenolic composition, essential oil, and antioxidant activity in the aerial part of Artemisia herba-alba from several provenances: A comparative study. Int. J. Food Prop. 2015, 19, 549–563. [Google Scholar] [CrossRef]
- Kordali, S.; Cakir, A.; Mavi, A.; Kilic, H.; Yildirim, A. Screening of chemical composition and antifungal and antioxidant activities of the essential oils from three turkish Artemisia species. J. Agric. Food Chem. 2005, 53, 1408–1416. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.J.; Huang, Y.M.; Chen, K.T.; Yan, Y.J.; Xu, L.; Liu, Y. Chemical composition and antimicrobial mechanism of essential oil from Dendranthema indicum var. Aromaticum. Food Sci. 2012, 33, 35–39. [Google Scholar]
- Wei, D.W.; Xu, M.M.; Sun, W.Y.; Jia, C.Y.; Zhang, X.W. Antioxidant activity of aqueous extracts from different organs of Opisthopappus shih. J. Chin. Inst. Food Sci. Technol. 2015, 15, 56–63. [Google Scholar]
- Yang, X.W.; Zou, L.; Wu, Q.; Fu, D.X. Studies on chemical constituents from whole plants of Crossostephium chinense. China J. Chin. Mater. Medica 2008, 33, 905–908. [Google Scholar]
- Móricz, Á.M.; Häbe, T.T.; Böszörményi, A.; Ott, P.G.; Morlock, G.E. Tracking and identification of antibacterial components in the essential oil of Tanacetum vulgare l. By the combination of high-performance thin-layer chromatography with direct bioautography and mass spectrometry. J. Chromatogr. A 2015, 1422, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Goudarzi, T.; Saharkhiz, M.J.; Rowshan, V. Ontogenetic variation of essential oil content and constituents in tansy (Tanacetum vulgare l.). J. Appl. Res. Med. Aromat. Plants 2015, 2, 48–53. [Google Scholar] [CrossRef]
- Lawrence, B.M. Essential oils as source of natural aroma chemicals. Perfum. Flavor 1992, 17, 15–28. [Google Scholar]
- Brown, A.M.G.; Edwards, C.M.; Davey, M.R.; Power, J.B.; Lowe, K.C. Effects of extracts of Tanacetum species on human polymorphonuclear leucocyte activity in vitro. Phytother. Res. 1997, 11, 479–484. [Google Scholar] [CrossRef]
- Piras, A.; Falconieri, D.; Bagdonaite, E.; Maxia, A.; Gonçalves, M.J.; Cavaleiro, C.; Salgueiro, L.; Porcedda, S. Chemical composition and antifungal activity of supercritical extract and essential oil of Tanacetum vulgare growing wild in lithuania. Nat. Prod. Res. 2014, 28, 1906–1909. [Google Scholar] [CrossRef] [PubMed]
- Baranauskienė, R.; Kazernavičiūtė, R.; Pukalskienė, M.; Maždžierienė, R.; Venskutonis, P.R. Agrorefinery of Tanacetum vulgare l. Into valuable products and evaluation of their antioxidant properties and phytochemical composition. Ind. Crops Prod. 2014, 60, 113–122. [Google Scholar] [CrossRef]
- Liu, X.C.; Li, Y.; Wang, T.; Wang, Q.; Liu, Z.L. Chemical composition and insecticidal activity of essential oil of Artemisia frigida willd (compositae) against two grain storage insects. Trop. J. Pharm. Res. 2014, 13, 587–592. [Google Scholar] [CrossRef]
- Liu, Z.L.; Chu, S.S.; Liu, Q.R. Chemical composition and insecticidal activity against Sitophilus zeamais of the essential oils of Artemisia capillaris and Artemisia mongolica. Molecules 2010, 15, 2600–2608. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.S.; Liu, Q.R.; Liu, Z.L. Insecticidal activity and chemical composition of the essential oil of Artemisia vestita from china against Sitophilus zeamais. Biochem. Syst. Ecol. 2010, 38, 489–492. [Google Scholar] [CrossRef]
- Chu, S.S.; Liu, Z.L.; Du, S.S.; Deng, Z.W. Chemical composition and insecticidal activity against Sitophilus zeamais of the essential oils derived from Artemisia giraldii and Artemisia subdigitata. Molecules 2012, 17, 7255–7265. [Google Scholar] [CrossRef] [PubMed]
- Isman, M.B. Plant essential oils as green pesticides for pest and disease management. In ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2004; Volume 887, pp. 41–51. [Google Scholar]
- Zhou, K.; Guo, W.M.; Xu, Y.C. Advances of research on allelopathic potential in compositae. Acta Ecol. Sin. 2004, 24, 1776–1784. [Google Scholar]
- Abrahim, D.; Braguini, W.L.; Kelmer-Bracht, A.M.; Ishii-Iwamoto, E.L. Effects of four monoterpenes on germination, primary root growth, and mitochondrial respiration of maize. J. Chem. Ecol. 2000, 26, 611–624. [Google Scholar] [CrossRef]
- Barney, J.N.; Hay, A.G.; Weston, L.A. Isolation and characterization of allelopathic volatiles from mugwort (Artemisia vulgaris). J. Chem. Ecol. 2005, 31, 247–265. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, J.T.; Tollsten, L.; Bergström, L.G. Floral scents—A checklist of volatile compounds isolated by head-space techniques. Phytochemistry 1993, 33, 253–280. [Google Scholar] [CrossRef]
- Xiao, Z.; Fan, B.; Niu, Y.; Wu, M.; Liu, J.; Ma, S. Characterization of odor-active compounds of various Chrysanthemum essential oils by gas chromatography-olfactometry, gas chromatography-mass spectrometry and their correlation with sensory attributes. J. Chromatogr. B 2016, 1009, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Unsicker, S.B.; Kunert, G.; Gershenzon, J. Protective perfumes: The role of vegetative volatiles in plant defense against herbivores. Curr. Opin. Plant Biol. 2009, 12, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Batish, D.R.; Singh, H.P.; Kohli, R.K.; Kaur, S. Eucalyptus essential oil as a natural pesticide. For. Ecol. Manag. 2008, 256, 2166–2174. [Google Scholar] [CrossRef]
- Chen, W.; Vermaak, I.; Viljoen, A. Camphor—A fumigant during the black death and a coveted fragrant wood in ancient egypt and babylon—A review. Molecules 2013, 18, 5434–5454. [Google Scholar] [CrossRef] [PubMed]
- Rasmann, S.; Köllner, T.G.; Degenhardt, J.; Hiltpold, I.; Toepfer, S.; Kuhlmann, U.; Gershenzon, J.; Turlings, T.C.J. Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 2005, 434, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Schnee, C.; Köllner, T.G.; Held, M.; Turlings, T.C.J.; Gershenzon, J.; Degenhardt, J. The products of a single maize sesquiterpene synthase form a volatile defense signal that attracts natural enemies of maize herbivores. Proc. NatL. Acad. Sci. USA 2006, 103, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Tholl, D.; Bohlmann, J.; Pichersky, E. The family of terpene synthases in plants: A mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. Plant J. 2011, 66, 212–229. [Google Scholar] [CrossRef] [PubMed]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, function and metabolic engineering of plant volatile organic compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.B.; Chen, F.D.; Chen, S.M.; Wu, G.S.; Guo, W.M. Molecular phylogeny of Chrysanthemum, Ajania and its allies (anthemideae, asteraceae) as inferred from nuclear ribosomal its and chloroplast trnl-f igs sequences. Plant Syst. Evol. 2010, 284, 153–169. [Google Scholar] [CrossRef]
- Radulovic, N.S.; Blagojevic, P.D.; Zlatkovic, B.K.; Palic, R.M. A gc/ms profile of the volatile constituents of the aerial parts of Artemisia abrotanum l. (asteraceae) from serbia. S. Afr. J. Chem. 2009, 62, 30–32. [Google Scholar]
- Van den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |






| Categories a | Number of Volatile Categories | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 b | S2 | S3 | S4 | S5 | S6 | S7 | S8 | S9 | S10 | S11 | S12 | S13 | S14 | |
| MT | 2 | 3 | 2 | 3 | 2 | 1 | 0 | 1 | 2 | 0 | 4 | 0 | 1 | 3 |
| ST | 9 | 2 | 4 | 13 | 4 | 3 | 0 | 7 | 4 | 5 | 8 | 3 | 2 | 8 |
| DT | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| OT | 6 | 7 | 3 | 17 | 1 | 20 | 4 | 12 | 7 | 3 | 7 | 5 | 6 | 20 |
| AC | 1 | 3 | 0 | 3 | 0 | 1 | 0 | 1 | 2 | 1 | 1 | 3 | 0 | 1 |
| FC | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 2 | 1 | 0 | 0 | 2 | 1 | 1 |
| Alkanes | 4 | 2 | 3 | 3 | 1 | 2 | 1 | 0 | 5 | 1 | 2 | 1 | 1 | 0 |
| Alkanols | 2 | 0 | 3 | 4 | 1 | 2 | 2 | 4 | 2 | 2 | 2 | 2 | 1 | 2 |
| Others | 2 | 2 | 6 | 12 | 1 | 7 | 4 | 8 | 11 | 4 | 4 | 6 | 5 | 5 |
| TC | 26 | 21 | 21 | 55 | 11 | 37 | 11 | 35 | 34 | 16 | 28 | 22 | 17 | 40 |
| Sample Number | Accessions | Collection Locality |
|---|---|---|
| S1 | A. sacrorum | Dalian, Liaoning province, China |
| S2 | A. absinthium | Chiba-shi, Japan |
| S3 | A. vulgaris ‘Variegate’ | Nanjing, Jiangsu province, China |
| S4 | A. yunnanensis | Yunnan province, China |
| S5 | A. japonica | Hiroshima, Japan |
| S6 | A. abrotanum | Chiba-shi, Japan |
| S7 | A. sericea | Nanjing, Jiangsu province, China |
| S8 | A. argyi | Jiangshan, Zhejiang province, China |
| S9 | T. vulgare | Tsukuba, Japan |
| S10 | C. indicum var. aromaticum | Shennongjia, Hubei province, China |
| S11 | C. indicum | Shennongjia, Hubei province, China |
| S12 | C. chinense | Xiamen, Fujian province, China |
| S13 | O. longilobus | Yuntai Mountain, Henan province, China |
| S14 | O. taihangensis | Yuntai Mountain, Henan province, China |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Li, X.; Jiang, Q.; Sun, H.; Jiang, J.; Chen, S.; Guan, Z.; Fang, W.; Chen, F. GC-MS Analysis of the Volatile Constituents in the Leaves of 14 Compositae Plants. Molecules 2018, 23, 166. https://doi.org/10.3390/molecules23010166
Wang Y, Li X, Jiang Q, Sun H, Jiang J, Chen S, Guan Z, Fang W, Chen F. GC-MS Analysis of the Volatile Constituents in the Leaves of 14 Compositae Plants. Molecules. 2018; 23(1):166. https://doi.org/10.3390/molecules23010166
Chicago/Turabian StyleWang, Yiguang, Xiran Li, Qinjie Jiang, Hainan Sun, Jiafu Jiang, Sumei Chen, Zhiyong Guan, Weimin Fang, and Fadi Chen. 2018. "GC-MS Analysis of the Volatile Constituents in the Leaves of 14 Compositae Plants" Molecules 23, no. 1: 166. https://doi.org/10.3390/molecules23010166
APA StyleWang, Y., Li, X., Jiang, Q., Sun, H., Jiang, J., Chen, S., Guan, Z., Fang, W., & Chen, F. (2018). GC-MS Analysis of the Volatile Constituents in the Leaves of 14 Compositae Plants. Molecules, 23(1), 166. https://doi.org/10.3390/molecules23010166