Host-Guest Interaction of Cucurbit[8]uril with N-(3-Aminopropyl)cyclohexylamine: Cyclohexyl Encapsulation Triggered Ternary Complex
Abstract
:1. Introduction
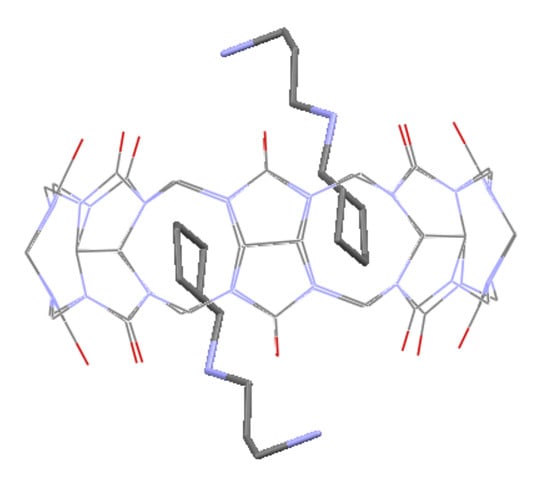
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Isothermal Titration Calorimetry (ITC)
3.3. Crystal Structure Determination
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hof, F.; Craig, S.L.; Nuckolls, C.; Rebek, J., Jr. Molecular encapsulation. Angew. Chem. Int. Ed. 2002, 41, 1488–1508. [Google Scholar] [CrossRef]
- Ko, Y.H.; Kim, E.; Hwang, I.; Kim, K. Supramolecular assemblies built with host-stabilized charge-transfer interactions. Chem. Commun. 2007, 13, 1305–1315. [Google Scholar] [CrossRef] [PubMed]
- Jordan, J.H.; Gibb, B.C. Molecular containers assembled through the hydrophobic effect. Chem. Soc. Rev. 2015, 44, 547–585. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Tang, X.; Yu, H.; Cai, Z.; Huang, Z.; Wang, D.; Xu, J.-F.; Zhang, X. Supramolecular catalyst functions in catalytic amount: Cucurbit[8]uril accelerates the photodimerization of Brooker’s merocyanine. Chem. Sci. 2017, 8, 8357–8361. [Google Scholar] [CrossRef]
- Biros, S.M.; Rebek, J., Jr. Structure and binding properties of water-soluble cavitands and capsules. Chem. Soc. Rev. 2007, 36, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.L.; Yang, H.; Wang, Z.Q.; Zhang, X. Cucurbit[8]uril-based supramolecular polymers. Chem. Asian J. 2013, 8, 1626–1632. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.-L.; Chen, S.; Yang, Y.; Tao, Z. Facile Cucurbit[8]uril-based supramolecular approach to fabricate tunable luminescent materials in aqueous solution. J. Am. Chem. Soc. 2016, 138, 6177–6183. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jung, I.S.; Kim, S.Y.; Lee, E.; Kang, J.K.; Sakamoto, S.; Yamaguchi, K.; Kim, K. New cucurbituril homologues: Syntheses, isolation, characterization, and X-ray crystal structures of cucurbit-[n]uril (n = 5, 7, and 8). J. Am. Chem. Soc. 2000, 122, 540–541. [Google Scholar] [CrossRef]
- Day, A.; Arnold, A.P.; Blanch, R.J.; Snushall, B. Controlling factors in the synthesis of cucurbituril and its homologues. J. Org. Chem. 2001, 66, 8094–8100. [Google Scholar] [CrossRef] [PubMed]
- Sindelar, V.; Cejas, M.A.; Raymo, F.M.; Chen, W.; Parker, S.E.; Kaifer, A.E. Supramolecular assembly of 2,7-dimethyldiazapyrenium and cucurbit[8]uril: A new fluorescent host for detection of catechol and dopamine. Chem. Eur. J. 2005, 11, 7054–7059. [Google Scholar] [CrossRef] [PubMed]
- Pattabiraman, M.; Natarajan, A.; Kaliappan, R.; Mague, J.T.; Ramamurthy, V. Template directed photodimerization of trans-1,2-bis(n-pyridyl)ethylenes and stilbazoles in water. Chem. Commun. 2005, 36, 4542–4544. [Google Scholar] [CrossRef] [PubMed]
- Bush, M.E.; Bouley, N.D.; Urbach, A.R. Charge-mediated recognition of N-terminal tryptophan in aqueous solution by a synthetic host. J. Am. Chem. Soc. 2005, 127, 14511–14517. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yu, Y.; Gao, J.; Wang, Z.; Zhang, X. Water-soluble supramolecular polymerization driven by multiple host-stabilized charge-transfer interactions. Angew. Chem. Int. Ed. 2010, 49, 6576–6579. [Google Scholar] [CrossRef] [PubMed]
- Appel, E.A.; Biedermann, F.; Rauwald, U.; Jones, S.T.; Zayed, J.M.; Scherman, O.A. Supramolecular Cross-linked networks via host-guest complexation with cucurbit[8]uril. J. Am. Chem. Soc. 2010, 132, 14251–14260. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.-D.; Tian, J.; Hanifi, D.; Zhang, Y.; Sue, A.C.-H.; Zhou, T.-Y.; Zhang, L.; Zhao, X.; Liu, Y.; Li, Z.-T. Toward a single-layer two-dimensional honeycomb supramolecular organic framework in water. J. Am. Chem. Soc. 2013, 135, 17913–17918. [Google Scholar] [CrossRef] [PubMed]
- Ko, Y.H.; Kim, H.; Kim, Y.; Kim, K. U-shaped conformation of alkyl chains bound to a synthetic host. Angew. Chem. Int. Ed. 2008, 47, 4106–4109. [Google Scholar] [CrossRef] [PubMed]
- Xiao, X.; Liu, J.-X.; Chen, K.; Zhu, Q.-J.; Xue, S.-F.; Tao, Z. Metal cation controlled supramolecular assembly of 1-butyl-4,4′-bipyridinium and cucurbit[8]uril. Eur. J. Inorg. Chem. 2010, 2010, 2956–2961. [Google Scholar] [CrossRef]
- Xiao, X.; Sun, N.; Qi, D.; Jiang, J. Unprecedented cucurbituril-based ternary host–guest supramolecular polymers mediated through included alkyl chains. Polym. Chem. 2014, 5, 5211–5217. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |




| Host-Guest | Ka/(L·mol−1) | n | ΔG/(kJ·mol−1) | ΔH/(kJ·mol−1) | TΔS/(kJ·mol−1) |
|---|---|---|---|---|---|
| Q[8]·CyA | (3.07 ± 0.02) × 106 | 0.504 | −37.02 ± 0.2 | −29.48 ± 0.2 | 7.54 ± 0.4 |
| Q[8]·NECyA | (1.27 ± 0.04) × 107 | 0.500 | −40.55 ± 0.6 | −33.96 ± 0.3 | 6.59 ± 0.3 |
| Q[8]·NCyEA | (4.59 ± 0.03) × 106 | 0.554 | −37.93 ± 0.4 | −28.67 ± 0.3 | 9.26 ± 0.2 |
| Q[8]·N3ACyA | (1.55 ± 0.02) × 107 | 0.475 | −41.03 ± 0.4 | −26.77 ± 0.2 | 14.26 ± 0.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, Y.; Wang, C.-Z.; Tian, M.; Tao, Z.; Ni, X.-L.; Prior, T.J.; Redshaw, C. Host-Guest Interaction of Cucurbit[8]uril with N-(3-Aminopropyl)cyclohexylamine: Cyclohexyl Encapsulation Triggered Ternary Complex. Molecules 2018, 23, 175. https://doi.org/10.3390/molecules23010175
Xia Y, Wang C-Z, Tian M, Tao Z, Ni X-L, Prior TJ, Redshaw C. Host-Guest Interaction of Cucurbit[8]uril with N-(3-Aminopropyl)cyclohexylamine: Cyclohexyl Encapsulation Triggered Ternary Complex. Molecules. 2018; 23(1):175. https://doi.org/10.3390/molecules23010175
Chicago/Turabian StyleXia, Yu, Chuan-Zeng Wang, Mengkui Tian, Zhu Tao, Xin-Long Ni, Timothy J. Prior, and Carl Redshaw. 2018. "Host-Guest Interaction of Cucurbit[8]uril with N-(3-Aminopropyl)cyclohexylamine: Cyclohexyl Encapsulation Triggered Ternary Complex" Molecules 23, no. 1: 175. https://doi.org/10.3390/molecules23010175
APA StyleXia, Y., Wang, C. -Z., Tian, M., Tao, Z., Ni, X. -L., Prior, T. J., & Redshaw, C. (2018). Host-Guest Interaction of Cucurbit[8]uril with N-(3-Aminopropyl)cyclohexylamine: Cyclohexyl Encapsulation Triggered Ternary Complex. Molecules, 23(1), 175. https://doi.org/10.3390/molecules23010175