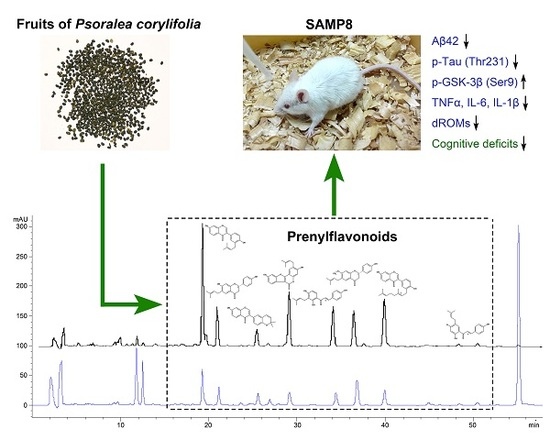
Dietary Total Prenylflavonoids from the Fruits of Psoralea corylifolia L. Prevents Age-Related Cognitive Deficits and Down-Regulates Alzheimer’s Markers in SAMP8 Mice
Abstract
:1. Introduction
2. Results
2.1. Yield and Chemical Composition of TPFB
2.2. TPFB Prevented Age-Related Cognitive Impairments of SAMP8 Mice
2.3. TPFB Reduced Aβ42 Accumulation, Tau Phosphorylation, and Glycogen Synthase Kinase 3β (GSK-3β) Activation in SAMP8 Brains
2.4. TPFB Decreased Brain Expression of TNF-α, IL-6, and IL-1β in SAMP8 Mice
2.5. TPFB Reduced Serum Oxidative Level in SAMP8 Mice
3. Discussion
4. Materials and Methods
4.1. Preparation and Quantification of the Total Prenylflavonoids from Buguzhi (TPFB)
4.2. Animals and Drug Intervention
4.3. Morris Water Maze Test (MWM)
4.4. Blood and Brain Tissue Collection
4.5. Western Blot
4.6. ELISA
4.7. Serum d-ROMs Test
4.8. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bronzuoli, M.R.; Iacomino, A.; Steardo, L.; Scuderi, C. Targeting neuroinflammation in Alzheimer’s disease. J. Inflamm. Res. 2016, 9, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s Association. Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2017, 13, 325–373. [Google Scholar]
- Funato, H.; Yoshimura, M.; Kusui, K.; Tamaoka, A.; Ishikawa, K.; Ohkoshi, N.; Namekata, K.; Okeda, R.; Ihara, Y. Quantitation of amyloid beta-protein (A beta) in the cortex during aging and in Alzheimer’s disease. Am. J. Pathol. 1998, 152, 1633–1640. [Google Scholar] [PubMed]
- Tam, J.H.; Pasternak, S.H. Amyloid and Alzheimer’s disease: Inside and out. Can. J. Neurol. Sci. 2012, 39, 286–298. [Google Scholar] [CrossRef] [PubMed]
- Kosik, K.S.; Joachim, C.L.; Selkoe, D.J. Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc. Natl. Acad. Sci. USA 1986, 83, 4044–4048. [Google Scholar] [CrossRef] [PubMed]
- Šimić, G.; BabićLeko, M.; Wray, S.; Harrington, C.; Delalle, I.; Jovanov-Milošević, N.; Bažadona, D.; Buée, L.; de Silva, R.; Di Giovanni, G.; et al. Tau protein hyperphosphorylation and aggregation in Alzheimer’s disease and other tauopathies, and possible neuroprotective Strategies. Biomolecules 2016, 6, 6. [Google Scholar] [CrossRef]
- Lv, Z.Y.; Tan, C.C.; Yu, J.T.; Tan, L. Spreading of pathology in Alzheimer’s disease. Neurotox. Res. 2017, 32, 707–722. [Google Scholar] [CrossRef] [PubMed]
- Bagyinszky, E.; Giau, V.V.; Shim, K.; Suk, K.; An, S.S.A.; Kim, S. Role of inflammatory molecules in the Alzheimer’s disease progression and diagnosis. J. Neurol. Sci. 2017, 376, 242–254. [Google Scholar] [CrossRef] [PubMed]
- Tönnies, E.; Trushina, E. Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. J. Alzheimer’s Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef] [PubMed]
- Chopra, B.; Dhingra, A.K.; Dhar, K.L. Psoralea corylifolia L. (Buguchi)—Folklore to modern evidence: Review. Fitoterapia 2013, 90, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, W.; Wang, Y.; Lu, J.; Chen, X. The chemical constituents and bioactivities of Psoralea corylifolia Linn.: A review. Am. J. Chin. Med. 2016, 44, 35–60. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Yon, G.H.; Hong, K.S.; Yoo, D.S.; Choi, C.W.; Park, W.K.; Kong, J.Y.; Kim, Y.S.; Ryu, S.Y. In vitro BACE-1 inhibitory phenolic components from the seeds of Psoralea corylifolia. Planta Med. 2008, 74, 1405–1408. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Yang, Y.; Zhang, Y. Isobavachalcone and bavachinin from Psoraleae Fructus modulate Aβ42 aggregation process through different mechanisms in vitro. FEBS Lett. 2013, 587, 2930–2935. [Google Scholar] [CrossRef] [PubMed]
- Pallas, M.; Camins, A.; Smith, M.A.; Perry, G.; Lee, H.G.; Casadesus, G. From aging to Alzheimer’s disease: Unveiling “the switch” with the senescence-accelerated mouse model (SAMP8). J. Alzheimer’s Dis. 2008, 15, 615–624. [Google Scholar] [CrossRef]
- Morley, J.E.; Armbrecht, H.J.; Farr, S.A.; Kumar, V.B. The senescence accelerated mouse (SAMP8) as a model for oxidative stress and Alzheimer’s disease. Biochim. Biophys. Acta 2012, 1822, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Farr, S.A.; Kumar, V.B.; Armbrecht, H.J. The SAMP8 mouse: A model to develop therapeutic interventions for Alzheimer’s disease. Curr. Pharm. Des. 2012, 18, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.R.; Zhou, W.X.; Zhang, Y.X. The behavioral, pathological and therapeutic features of the senescence-accelerated mouse prone 8 strain as an Alzheimer’s disease animal model. Ageing Res. Rev. 2014, 13, 13–37. [Google Scholar] [CrossRef] [PubMed]
- Porquet, D.; Casadesús, G.; Bayod, S.; Vicente, A.; Canudas, A.M.; Vilaplana, J.; Pelegrí, C.; Sanfeliu, C.; Camins, A.; Pallàs, M.; et al. Dietary resveratrol prevents Alzheimer’s markers and increases life span in SAMP8. AGE 2013, 35, 1851–1865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beurel, E.; Grieco, S.F.; Jope, R.S. Glycogen synthase kinase-3 (GSK3): Regulation, actions, and diseases. Pharmacol. Ther. 2015, 148, 114–131. [Google Scholar] [CrossRef] [PubMed]
- Botta, B.; Menendez, P.; Zappia, G.; de Lima, R.A.; Torge, R.; Monachea, G.D. Prenylated isoflavonoids: Botanical distribution, structures, biological activities and biotechnological studies. An update (1995–2006). Curr. Med. Chem. 2009, 16, 3414–3468. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Jiang, Y.; Yang, J.; He, J.; Sun, J.; Chen, F.; Zhang, M.; Yang, B. Prenylated flavonoids, promising nutraceuticals with impressive biological activities. Trends Food Sci. Technol. 2015, 44, 93–104. [Google Scholar] [CrossRef]
- Ginés, C.; Cuesta, S.; Kireev, R.; García, C.; Rancan, L.; Paredes, S.D.; Vara, E.; Tresguerres, J.A. Protective effect of resveratrol against inflammation, oxidative stress and apoptosis in pancreas of aged SAMP8 mice. Exp. Gerontol. 2017, 90, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Palomera-Ávalos, V.; Griñán-Ferré, C.; Puigoriol-Ilamola, D.; Camins, A.; Sanfeliu, C.; Canudas, A.M.; Pallàs, M. Resveratrol protects SAMP8 brain under metabolic stress: Focus on mitochondrial function and Wnt Pathway. Mol. Neurobiol. 2017, 54, 1661–1676. [Google Scholar] [CrossRef] [PubMed]
- Palomera-Ávalos, V.; Griñán-Ferré, C.; Izquierdo, V.; Camins, A.; Sanfeliu, C.; Pallàs, M. Metabolic stress induces cognitive disturbances and inflammation in aged mice: Protective role of resveratrol. Rejuvenation Res. 2017, 20, 202–217. [Google Scholar] [CrossRef] [PubMed]
- Gacar, N.; Mutlu, O.; Utkan, T.; Celikyurt, I.K.; Gocmez, S.S.; Ulak, G. Beneficial effects of resveratrol on scopolamine but not mecamylamine induced memory impairment in the passive avoidance and Morris water maze tests in rats. Pharmacol. Biochem. Behav. 2011, 99, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Gupta, L.K.; Mediratta, P.K.; Bhattacharya, S.K. Effect of resveratrol on scopolamine-induced cognitive impairment in mice. Pharmacol. Rep. 2012, 64, 438–444. [Google Scholar] [PubMed]
- Ma, T.; Tan, M.S.; Yu, J.T.; Tan, L. Resveratrol as a therapeutic agent for Alzheimer’s disease. Biomed. Res. Int. 2014, 2014, 350516. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.S.; Thomas, R.G.; Craft, S.; van Dyck, C.H.; Mintzer, J.; Reynolds, B.A.; Brewer, J.B.; Rissman, R.A.; Raman, R.; Aisen, P.S. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology 2015, 85, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Moussa, C.; Hebron, M.; Huang, X.; Ahn, J.; Rissman, R.A.; Aisen, P.S.; Turner, R.S. Resveratrol regulates neuro-inflammation and induces adaptive immunity in Alzheimer’s disease. J. Neuroinflamm. 2017, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Jing, H.; Wang, S.; Wang, M.; Fu, W.; Zhang, C.; Xu, D. Isobavachalcone attenuates MPTP-induced Parkinson’s disease in mice by inhibition of microglial activation through NF-κB Pathway. PLoS ONE 2017, 12, e0169560. [Google Scholar] [CrossRef] [PubMed]
- Hung, Y.L.; Fang, S.H.; Wang, S.C.; Cheng, W.C.; Liu, P.L.; Su, C.C.; Chen, C.S.; Huang, M.Y.; Hua, K.F.; Shen, K.H.; et al. Corylin protects LPS-induced sepsis and attenuates LPS-induced inflammatory response. Sci. Rep. 2017, 7, 46299. [Google Scholar] [CrossRef] [PubMed]
- Szliszka, E.; Skaba, D.; Czuba, Z.P.; Krol, W. Inhibition of inflammatory mediators by neobavaisoflavone in activated RAW264.7 macrophages. Molecules 2011, 16, 3701–3712. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Li, G.; Chen, L.; Zhang, Z.; Yin, J.J.; Wu, T.; Cheng, Z.; Wei, X.; Wang, Z. Isolation of antioxidants from Psoralea corylifolia fruits using high-speed counter-current chromatography guided by thin layer chromatography-antioxidant autographic assay. J. Chromatogr. A 2002, 1217, 5470–5476. [Google Scholar] [CrossRef] [PubMed]
- Haraguchi, H.; Inoue, J.; Tamura, Y.; Mizutani, K. Antioxidative components of Psoralea corylifolia (Leguminosae). Phytother. Res. 2002, 16, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Farr, S.A.; Ripley, J.L.; Sultana, R.; Zhang, Z.; Niehoff, M.L.; Platt, T.L.; Murphy, M.P.; Morley, J.E.; Kumar, V.; Butterfield, D.A. Antisense oligonucleotide against GSK-3β in brain of SAMP8 mice improves learning and memory and decreases oxidative stress: Involvement of transcription factor Nrf2 and implications for Alzheimer disease. Free Radic. Biol. Med. 2014, 67, 387–395. [Google Scholar] [CrossRef] [PubMed]
- Engel, T.; Hernández, F.; Avila, J.; Lucas, J.J. Full reversal of Alzheimer’s disease-like phenotype in a mouse model with conditional overexpression of glycogen synthase kinase-3. J. Neurosci. 2006, 26, 5083–5090. [Google Scholar] [CrossRef] [PubMed]
- Llorens-Martín, M.; Jurado, J.; Hernández, F.; Avila, J. GSK-3β, a pivotal kinase in Alzheimer disease. Front. Mol. Neurosci. 2014, 7, 46. [Google Scholar] [CrossRef]
- Maqbool, M.; Mobashir, M.; Hoda, N. Pivotal role of glycogen synthase kinase-3: A therapeutic target for Alzheimer’s disease. Eur. J. Med. Chem. 2016, 107, 63–81. [Google Scholar] [CrossRef] [PubMed]
- Gameiro, I.; Michalska, P.; Tenti, G.; Cores, Á.; Buendia, I.; Rojo, A.I.; Georgakopoulos, N.D.; Hern#xE1ndez-Guijo, J.M.; Teresa Ramos, M.; Wells, G.; et al. Discovery of the first dual GSK3β inhibitor/Nrf2 inducer. A new multitarget therapeutic strategy for Alzheimer’s disease. Sci. Rep. 2017, 7, 45701. [Google Scholar] [CrossRef] [PubMed]
- Omar, S.H.; Scott, C.J.; Hamlin, A.S.; Obied, H.K. The protective role of plant biophenols in mechanisms of Alzheimer’s disease. J. Nutr. Biochem. 2017, 47, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Tomé-Carneiro, J.; Larrosa, M.; González-Sarrías, A.; Tomás-Barberán, F.A.; García-Conesa, M.T.; Espín, J.C. Resveratrol and clinical trials: The crossroad from in vitro studies to human evidence. Curr. Pharm. Des. 2013, 19, 6064–6093. [Google Scholar] [CrossRef] [PubMed]
- Puksasook, T.; Kimura, S.; Tadtong, S.; Jiaranaikulwanitch, J.; Pratuangdejkul, J.; Kitphati, W.; Suwanborirux, K.; Saito, N.; Nukoolkarn, V. Semisynthesis and biological evaluation of prenylated resveratrol derivatives as multi-targeted agents for Alzheimer’s disease. J. Nat. Med. 2017, 71, 665–682. [Google Scholar] [CrossRef] [PubMed]
- Brents, L.K.; Medina-Bolivar, F.; Seely, K.A.; Nair, V.; Bratton, S.M.; Nopo-Olazabal, L.; Patel, R.Y.; Liu, H.; Doerksen, R.J.; Prather, P.L.; et al. Natural prenylated resveratrol analogs arachidin-1 and -3 demonstrate improved glucuronidation profiles and have affinity for cannabinoid receptors. Xenobiotica 2012, 42, 139–156. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.F.; Zhang, Y.B.; Chen, Z.J.; Zhang, Y.T.; Yang, X.W. Plasma pharmacokinetics and cerebral nuclei distribution of major constituents of Psoraleae Fructus in rats after oral administration. Phytomedicine 2018, 38, 166–174. [Google Scholar] [CrossRef]
- Ahmed, T.; Javed, S.; Javed, S.; Tariq, A.; Šamec, D.; Tejada, S.; Nabavi, S.F.; Braidy, N.; Nabavi, S.M. Resveratrol and Alzheimer’s Disease: Mechanistic Insights. Mol. Neurobiol. 2017, 54, 2622–2635. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yuan, J.; Pang, J.; Ma, J.; Han, B.; Geng, Y.; Shen, L.; Wang, H.; Ma, Q.; Wang, Y.; et al. Effects of Chronic Stress on Cognition in Male SAMP8 Mice. Cell. Physiol. Biochem. 2016, 39, 1078–1086. [Google Scholar] [CrossRef] [PubMed]
- Tong, J.J.; Chen, G.H.; Wang, F.; Li, X.W.; Cao, L.; Sui, X.; Tao, F.; Yan, W.W.; Wei, Z.J. Chronic acarbose treatment alleviates age-related behavioral and biochemical changes in SAMP8 mice. Behav. Brain Res. 2015, 284, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, I.; Morishita, Y.; Imai, K.; Nakamura, M.; Nakachi, K.; Hayashi, T. High-throughput spectrophotometric assay of reactive oxygen species in serum. Mutat. Res. 2007, 631, 55–61. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |




© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.-J.; Yang, Y.-F.; Zhang, Y.-T.; Yang, D.-H. Dietary Total Prenylflavonoids from the Fruits of Psoralea corylifolia L. Prevents Age-Related Cognitive Deficits and Down-Regulates Alzheimer’s Markers in SAMP8 Mice. Molecules 2018, 23, 196. https://doi.org/10.3390/molecules23010196
Chen Z-J, Yang Y-F, Zhang Y-T, Yang D-H. Dietary Total Prenylflavonoids from the Fruits of Psoralea corylifolia L. Prevents Age-Related Cognitive Deficits and Down-Regulates Alzheimer’s Markers in SAMP8 Mice. Molecules. 2018; 23(1):196. https://doi.org/10.3390/molecules23010196
Chicago/Turabian StyleChen, Zhi-Jing, Yan-Fang Yang, Ying-Tao Zhang, and Dong-Hui Yang. 2018. "Dietary Total Prenylflavonoids from the Fruits of Psoralea corylifolia L. Prevents Age-Related Cognitive Deficits and Down-Regulates Alzheimer’s Markers in SAMP8 Mice" Molecules 23, no. 1: 196. https://doi.org/10.3390/molecules23010196
APA StyleChen, Z. -J., Yang, Y. -F., Zhang, Y. -T., & Yang, D. -H. (2018). Dietary Total Prenylflavonoids from the Fruits of Psoralea corylifolia L. Prevents Age-Related Cognitive Deficits and Down-Regulates Alzheimer’s Markers in SAMP8 Mice. Molecules, 23(1), 196. https://doi.org/10.3390/molecules23010196