Antioxidant and Fluorescence Properties of Hydrogenolyzised Polymeric Proanthocyanidins Prepared Using SO42−/ZrO2 Solid Superacids Catalyst
Abstract
:1. Introduction
2. Results and Analysis
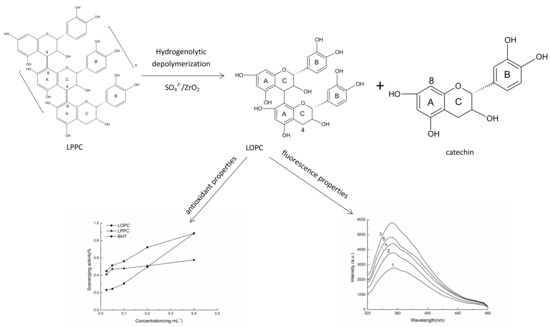
2.1. Depolymerization of LPPC
2.2. Detection and Analysis of LPPC and LOPC Structures
2.3. Antioxidant Properties of LOPC
2.3.1. Reducing Power
2.3.2. DPPH• Scavenging Activity
2.3.3. ABTS•+ Scavenging Activity
2.4. Fluorescence Characteristics of LOPC
2.4.1. Effect of Solvent Polarity on Fluorescence Characteristics of LOPC
2.4.2. Effect of pH on Fluorescence Characteristics of LOPC
2.4.3. Effects of Different Metal Ions on Fluorescence Properties of LOPC
3. Materials and methods
3.1. Experimental Materials, Reagents and Instruments
3.2. Sample Preparation
3.2.1. Preparation of LPPC
3.2.2. Preparation of SO42−/ZrO2 Solid Superacid Catalyst
3.2.3. Depolymerization of LPPC
3.3. Characterization of Proanthocyanidins
3.3.1. Determination of Average Degree of Polymerization and Degradation Rate
3.3.2. Molecular Weight Distribution
3.3.3. Spectral Analysis
3.4. Determination of Antioxidant Activity of Proanthocyanidins
3.5. Determination of Fluorescent Properties of Proanthocyanidins
3.5.1. Determination of Optimal Wavelength of Excitation Light
3.5.2. Effects of Different Metal Ions on Fluorescent Properties of Degraded Proanthocyanidins
3.5.3. Fluorescent Properties of LOPC
3.5.3.1 Effect of Solvent Polarity on Fluorescent Properties of LOPC
3.5.3.2 Effect of pH on Fluorescent Properties of LOPC
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Porter, L.J.; Newman, R.H.; Foo, L.Y.; Wong, H.; Hemingway, R.W. Polymeric proanthocyanidins. 13 C NMR studies of procyanidins. J. Chem. Soc. Perkin Trans. 1982, 1, 1217–1221. [Google Scholar] [CrossRef]
- Pekić, B.; Kovač, V.; Alonso, E.; Revilla, E. Study of the extraction of proanthocyanidins from grape seeds. Food Chem. 1998, 61, 201–206. [Google Scholar] [CrossRef]
- Hemingway, R.W.; Karchesy, J.J. Chemistry and Significance of Condensed Tannins; Springer Science & Business Media: Berlin, Germany, 2012. [Google Scholar]
- Du, X.; Lu, Z.; Tao, Y.; Liao, X.; Shi, B. Study on Antioxidant Activity of Catalytic Hydrogenation Products of Larch Polyproanthocyanidins. J. Sichuan Univ. 2005, 6, 68–73. [Google Scholar]
- Gabetta, B.; Fuzzati, N.; Griffini, A.; Lolla, E.; Pace, R.; Ruffilli, T.; Peterlongo, F. Characterization of proanthocyanidins from grape seeds. Fitoterapia 2000, 71, 162–175. [Google Scholar] [CrossRef]
- Jiang, G. Study on Fractionation Purification and Catalytic Degradation of Proanthocyanidins from Larch Bark. Ph.D. Thesis, Northeast Forestry University, Heilongjiang, China, 2013. [Google Scholar]
- Ma, Z.; Liu, C.; Niu, N.; Chen, Z.; Li, S.; Liu, S.; Li, J. Seeking Brightness from Nature: J-Aggregation-Induced Emission in Cellulolytic Enzyme Lignin Nanoparticles. ACS Sustain. Chem. Eng. 2018, 6, 3169–3175. [Google Scholar] [CrossRef]
- Rahimi, A.; Azarpira, A.; Kim, H.; Ralph, J.; Stahl, S.S. Chemoselective metal-free aerobic alcohol oxidation in lignin. J. Am. Chem. Soc. 2013, 135, 6415–6418. [Google Scholar] [CrossRef] [PubMed]
- Foo, L.A.I.Y. Polymeric proanthocyanidins of Photinia glabrescens, modification of molecular weight and nature of products from hydrogenolysis. Phytochemistry 1982, 21, 1741–1746. [Google Scholar]
- Li, C.; Lu, J.; Wei, S.; Chen, X.; Shi, Y.; Yong, K. Hydrogenation Degradation Process of Cinnamon High Polyproanthocyanidins. Nat. Prod. Res. Dev. 2009, 21, 660–663. [Google Scholar]
- Qin, Y.; Tian, H.; Zhang, J.; Yu, Y. Study on Antioxidant Activity of Degradation Products of Proanthocyanidins from Pinus yunnanensis. J. Southwest For. Univ. 2011, 31, 77–79. [Google Scholar]
- Wu, D. Study on Degradation and Antioxidation of Proanthocyanidins from Raspberry Seeds. Master Thesis, Shenyang Agricultural University, Liaoning, China, 2014. [Google Scholar]
- Du, X.; Tang, W.; Liao, X.; Shi, B. Pd/C catalytic hydrogenolysis of larch poly-proanthocyanidins. Chem. Res. Appl. 2005, 17, 456–459. [Google Scholar]
- Zhang, S.; Liu, L.; Ma, Y.; Fang, G. Antioxidant properties of SO42−/ZrO2 catalyzed hydrogen reduction of alkali lignin. J. Funct. Mater. 2014, 45, 13071–13075. [Google Scholar]
- Zhang, L.; Xu, M.; Wang, Y.; Hu, X. Determination of anthocyanin-metal ion complexation reaction of sorghum by fluorescence quenching method. J. For. Eng. 2016, 1, 58–63. [Google Scholar]
- Yang, L.; Han, H. The Softness and Hardness of Acid in the Theory of Pattern Recognition by Soft and Hard Acids. J. Qinghai Norm. Univ. 1998, 1, 34–37. [Google Scholar]
- Hider, R.C.; Liu, Z.D.; Khodr, H.H. Metal Chelation of Polyphenols. Methods Enzymol. 2001, 335, 190–203. [Google Scholar] [PubMed]
- Zheng, W.; Wang, S.Y. Oxygen radical absorbing capacity of phenolics in blueberries, cranberries, chokeberries and lingonberries. J. Agric. Food Chem. 2003, 51, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Blankenhorn, P.R.; Rishel, L.E.; Murphey, W.K.; Kline, D.E. Some Mechanical Properties of Impregnated Bark Board. For. Prod. J. 1977, 27, 31–38. [Google Scholar]
- Ouchemoukh, S.; Hachoud, S.; Boudraham, H.; Mokrani, A.; Louaileche, H. Antioxidant activities of some dried fruits consumed in Algeria. Food Sci. Technol. 2012, 49, 329–332. [Google Scholar] [CrossRef]
- Sánchez-Moreno, C.; Larrauri, J.A.; Saura-Calixto, F. Free radical scavenging capacity and inhibition of lipid oxidation of wines, grape juices and related polyphenolic constituents. Food Res. Int. 1999, 32, 407–412. [Google Scholar] [CrossRef]
- Yuan, X.; Gao, M.; Xiao, H.; Tan, C.; Du, Y. Free radical scavenging activities and bioactive substances of Jerusalem artichoke (Helianthus tuberosus L.) leaves. Food Chem. 2012, 133, 10–14. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |














| Complex | Metal Ion Concentration (μmol/L) | Maximum Emission Wavelength of Complex (nm) | Complexation Constant Ksv (× 106 L/mol) | ||
|---|---|---|---|---|---|
| Al3+-PC | ≤ 0.15 | 350 | 1.02 | 0.99967 | 0.00174 |
| Cu2+-PC | ≤ 0.15 | 350 | 8.31 | 0.99973 | 0.00335 |
| Ba2+-PC | ≤ 0.15 | 350 | 2.16 | 0.99987 | 0.00076 |
| Fe3+-PC | ≤ 0.15 | 350 | 21.05 | 0.99422 | 0.37551 |
| Ni2+-PC | ≤ 0.15 | 350 | 2.115 | 0.99694 | 0.01984 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ni, L.; Zhao, F.; Li, B.; Wei, T.; Guan, H.; Ren, S. Antioxidant and Fluorescence Properties of Hydrogenolyzised Polymeric Proanthocyanidins Prepared Using SO42−/ZrO2 Solid Superacids Catalyst. Molecules 2018, 23, 2445. https://doi.org/10.3390/molecules23102445
Ni L, Zhao F, Li B, Wei T, Guan H, Ren S. Antioxidant and Fluorescence Properties of Hydrogenolyzised Polymeric Proanthocyanidins Prepared Using SO42−/ZrO2 Solid Superacids Catalyst. Molecules. 2018; 23(10):2445. https://doi.org/10.3390/molecules23102445
Chicago/Turabian StyleNi, Liwen, Fanbin Zhao, Bolun Li, Tong Wei, Hang Guan, and Shixue Ren. 2018. "Antioxidant and Fluorescence Properties of Hydrogenolyzised Polymeric Proanthocyanidins Prepared Using SO42−/ZrO2 Solid Superacids Catalyst" Molecules 23, no. 10: 2445. https://doi.org/10.3390/molecules23102445
APA StyleNi, L., Zhao, F., Li, B., Wei, T., Guan, H., & Ren, S. (2018). Antioxidant and Fluorescence Properties of Hydrogenolyzised Polymeric Proanthocyanidins Prepared Using SO42−/ZrO2 Solid Superacids Catalyst. Molecules, 23(10), 2445. https://doi.org/10.3390/molecules23102445