Gene Delivery into the Inner Ear and Its Clinical Implications for Hearing and Balance
Abstract
:1. Introduction
2. Results
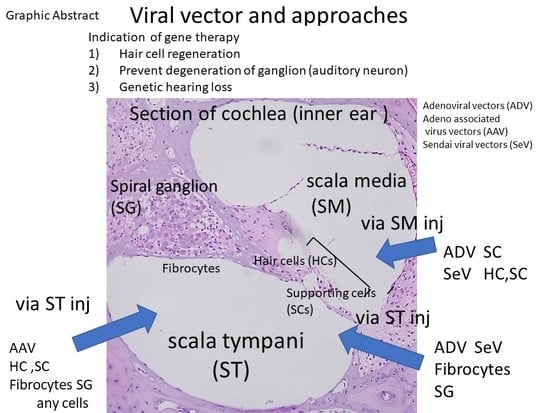
2.1. Anatomy of the Inner Ear and Gene Delivery
2.2. Advantages and Disadvantages of Administering Vectors into the Inner Ear
2.3. Regeneration of the Sensory Hair Cells
2.4. Preventing Degeneration of Inner Ear Spiral Neurons and Neuron Regeneration
2.5. Development of Endoscope for Topical Administration of Transgenes into Inner Ear
2.6. Problems Associated with Inner Ear Gene Delivery for Clinical Use
2.7. Genetic Hearing Loss
3. Conclusions
Funding
Conflicts of Interest
References
- World health Organization. Deafness and Hearing Loss. Available online: www.who.int/en/news-room/fact-sheets/detail/deafness-and-hearing-loss (accessed on 24 May 2018).
- Livingston, G.; Sommerlad, A.; Orgeta, V.; Costafreda, S.G.; Huntley, J.; Ames, D.; Ballard, C.; Banerjee, S.; Burns, A.; Cohen-Mansfield, J.; et al. Dementia prevention, intervention, and care. Lancet 2017, 390, 2673–2734. [Google Scholar] [CrossRef] [Green Version]
- Izumikawa, M.; Minoda, R.; Kawamoto, K.; Abrashkin, K.A.; Swiderski, D.L.; Dolan, D.F.; Brough, D.E.; Raphael, Y. Auditory hair cell replacement and hearing improvement by Atoh1 gene therapy in deaf mammals. Nat. Med. 2005, 11, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Woolf, N.K. The Role of Viral Infection in the Development of Otopathology: Labyrinthitis and Autoimmune Disease; Springer Verlag: New York, NY, USA, 1996; pp. 155–198. [Google Scholar]
- Ishimoto, S.; Kawamoto, K.; Kanzaki, S.; Raphael, Y. Gene transfer into supporting cells of the organ of Corti. Hear. Res. 2002, 173, 187–197. [Google Scholar] [CrossRef]
- Kanzaki, S.; Ogawa, K.; Camper, S.A.; Raphael, Y. Transgene expression in neonatal mouse inner ear explants mediated by first and advanced generation adenovirus vectors. Hear. Res. 2002, 169, 112–120. [Google Scholar] [CrossRef]
- Raphael, Y.; Frisancho, J.C.; Roessler, B.J. Adenoviral-mediated gene transfer into guinea pig cochlear cells in vivo. Neurosci. Lett. 1996, 207, 137–141. [Google Scholar] [CrossRef]
- Derby, M.L.; Sena-Esteves, M.; Breakefield, X.O.; Corey, D.P. Gene transfer into the mammalian inner ear using HSV-1 and vaccinia virus vectors. Hear. Res. 1999, 134, 1–8. [Google Scholar] [CrossRef]
- Han, J.J.; Mhatre, A.N.; Wareing, M.; Pettis, R.; Gao, W.Q.; Zufferey, R.N.; Trono, D.; Lalwani, A.K. Transgene expression in the guinea pig cochlea mediated by a lentivirus-derived gene transfer vector. Hum. Gene Ther. 1999, 10, 1867–1873. [Google Scholar] [CrossRef] [PubMed]
- Kanzaki, S.; Shiotani, A.; Inoue, M.; Hasegawa, M.; Ogawa, K. Sendai virus vector-mediated transgene expression in the cochlea in vivo. Audiol. Neurootol. 2007, 12, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Lalwani, A.K.; Walsh, B.J.; Reilly, P.G.; Carvalho, G.J.; Zolotukhin, S.; Muzyczka, N.; Mhatre, A.N. Long-term in vivo cochlear transgene expression mediated by recombinant adeno-associated virus. Gene Ther. 1998, 5, 277–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iizuka, T.; Kanzaki, S.; Mochizuki, H.; Inoshita, A.; Narui, Y.; Furukawa, M.; Kusunoki, T.; Saji, M.; Ogawa, K.; Ikeda, K. Noninvasive in vivo delivery of transgene via adeno-associated virus into supporting cells of the neonatal mouse cochlea. Hum. Gene Ther. 2008, 19, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Yang, H.; Colosi, P. Effect of genome size on AAV vector packaging. Mol. Ther. 2010, 18, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Bett, A.J.; Prevec, L.; Graham, F.L. Packaging capacity and stability of human adenovirus type 5 vectors. J. Virol. 1993, 67, 5911–5921. [Google Scholar] [PubMed]
- Weiss, M.A.; Frisancho, J.C.; Roessler, B.J.; Raphael, Y. Viral-mediated gene transfer in the cochlea. Int. J. Dev. Neurosci. 1997, 15, 577–583. [Google Scholar]
- Shu, Y.; Tao, Y.; Wang, Z.; Tang, Y.; Li, H.; Dai, P.; Gao, G.; Chen, Z.-Y. Identification of adeno-associated viral vectors that target neonatal and adult mammalian inner ear cell subtypes. Hum. Gene Ther. 2016, 27, 687–699. [Google Scholar] [CrossRef] [PubMed]
- Lalwani, A.K.; Mhatre, A.N. Cochlear gene therapy. Adv Otorhinolaryngol. 2000, 56, 275–278. [Google Scholar]
- Adler, H.J.; Raphael, Y. New hair cells arise from supporting cell conversion in the acoustically damaged chick inner ear. Neurosci. Lett. 1996, 205, 17–20. [Google Scholar] [CrossRef]
- McLean, W.J.; McLean, D.T.; Eatock, R.A.; Edge, A.S. Distinct capacity for differentiation to inner ear cell types by progenitor cells of the cochlea and vestibular organs. Development 2016, 143, 4381–4393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowenheim, H.; Furness, D.N.; Kil, J.; Zinn, C.; Gultig, K.; Fero, M.L.; Frost, D.; Gummer, A.W.; Roberts, J.M.; Rubel, E.W.; et al. Gene disruption of p27(Kip1) allows cell proliferation in the postnatal and adult organ of corti. Proc. Natl. Acad. Sci. USA 1999, 96, 4084–4088. [Google Scholar] [CrossRef] [PubMed]
- Kanzaki, S.; Beyer, L.A.; Swiderski, D.L.; Izumikawa, M.; Stover, T.; Kawamoto, K.; Raphael, Y. p27(Kip1) deficiency causes organ of Corti pathology and hearing loss. Hear. Res. 2006, 214, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, J.J.; Levine, E.M.; Zindy, F.; Goloubeva, O.; Roussel, M.F.; Smeyne, R.J. The cyclin-dependent kinase inhibitors p19(Ink4d) and p27(Kip1) are coexpressed in select retinal cells and act cooperatively to control cell cycle exit. Mol. Cell. Neurosci. 2002, 19, 359–374. [Google Scholar] [CrossRef] [PubMed]
- Sage, C.; Huang, M.; Karimi, K.; Gutierrez, G.; Vollrath, M.A.; Zhang, D.S.; Garcia-Anoveros, J.; Hinds, P.W.; Corwin, J.T.; Corey, D.P.; et al. Proliferation of functional hair cells in vivo in the absence of the retinoblastoma protein. Science 2005, 307, 1114–1118. [Google Scholar] [CrossRef] [PubMed]
- Jansson, L.; Kim, G.S.; Cheng, A.G. Making sense of Wnt signaling-linking hair cell regeneration to development. Front. Cell. Neurosci. 2015, 9, 66. [Google Scholar] [CrossRef] [PubMed]
- Bermingham, N.A.; Hassan, B.A.; Price, S.D.; Vollrath, M.A.; Ben-Arie, N.; Eatock, R.A.; Bellen, H.J.; Lysakowski, A.; Zoghbi, H.Y. Math1: An essential gene for the generation of inner ear hair cells. Science 1999, 284, 1837–1841. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.L.; Gao, W.Q. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat. Neurosci. 2000, 3, 580–586. [Google Scholar] [CrossRef] [PubMed]
- Kanzaki, S.; Stover, T.; Kawamoto, K.; Prieskorn, D.M.; Altschuler, R.A.; Miller, J.M.; Raphael, Y. Glial cell line-derived neurotrophic factor and chronic electrical stimulation prevent VIII cranial nerve degeneration following denervation. J. Comp. Neurol. 2002, 454, 350–360. [Google Scholar] [CrossRef] [PubMed]
- Seyyedi, M.; Viana, L.M.; Nadol, J.B., Jr. Within-subject comparison of word recognition and spiral ganglion cell count in bilateral cochlear implant recipients. Otol. Neurotol. 2014, 35, 1446–1450. [Google Scholar] [CrossRef] [PubMed]
- Pinyon, J.L.; Tadros, S.F.; Froud, K.E.; AC, Y.W.; Tompson, I.T.; Crawford, E.N.; Ko, M.; Morris, R.; Klugmann, M.; Housley, G.D. Close-field electroporation gene delivery using the cochlear implant electrode array enhances the bionic ear. Sci. Transl. Med. 2014, 6, 233ra254. [Google Scholar] [CrossRef] [PubMed]
- Silverstein, H.; Rowan, P.T.; Olds, M.J.; Rosenberg, S.I. Inner ear perfusion and the role of round window patency. Am. J. Otol. 1997, 18, 586–589. [Google Scholar] [PubMed]
- Kanzaki, S.; Saito, H.; Inoue, Y.; Ogawa, K. A new device for delivering drugs into the inner ear: Otoendoscope with microcatheter. Auris Nasus Larynx 2012, 39, 208–211. [Google Scholar] [CrossRef] [PubMed]
- van Egmond, S.L.; Visser, F.; Pameijer, F.A.; Grolman, W. Ex vivo and in vivo imaging of the inner ear at 7 Tesla MRI. Otol. Neurotol. 2014, 35, 725–729. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.S.; Xia, A.; Yuan, T.; Raphael, P.D.; Shelton, R.L.; Applegate, B.E.; Oghalai, J.S. Quantitative imaging of cochlear soft tissues in wild-type and hearing-impaired transgenic mice by spectral domain optical coherence tomography. Opt. Express 2011, 19, 15415–15428. [Google Scholar] [CrossRef] [PubMed]
- Pandya, A. Genetic hearing loss: The journey of discovery to destination—How close are we to therapy? Mol. Genet. Genom. Med. 2016, 4, 583–587. [Google Scholar] [CrossRef] [PubMed]
- Kanzaki, S.; Beyer, L.; Karolyi, I.J.; Dolan, D.F.; Fang, Q.; Probst, F.J.; Camper, S.A.; Raphael, Y. Transgene correction maintains normal cochlear structure and function in 6-month-old Myo15a mutant mice. Hear. Res. 2006, 214, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Probst, F.J.; Fridell, R.A.; Raphael, Y.; Saunders, T.L.; Wang, A.; Liang, Y.; Morell, R.J.; Touchman, J.W.; Lyons, R.H.; Noben-Trauth, K.; et al. Correction of deafness in shaker-2 mice by an unconventional myosin in a BAC transgene [see comments]. Science 1998, 280, 1444–1447. [Google Scholar] [CrossRef] [PubMed]
- Iizuka, T.; Kamiya, K.; Gotoh, S.; Sugitani, Y.; Suzuki, M.; Noda, T.; Minowa, O.; Ikeda, K. Perinatal Gjb2 gene transfer rescues hearing in a mouse model of hereditary deafness. Hum. Mol. Genet. 2015, 24, 3651–3661. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Askew, C.; Galvin, A.; Heman-Ackah, S.; Asai, Y.; Indzhykulian, A.A.; Jodelka, F.M.; Hastings, M.L.; Lentz, J.J.; Vandenberghe, L.H.; et al. Gene therapy restores auditory and vestibular function in a mouse model of Usher syndrome type 1c. Nat. Biotechnol. 2017, 35, 264–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Viral Vector | Animal | Transfected Cells | RW Penetration | References | |
|---|---|---|---|---|---|
| via SM | via RW | ||||
| ADV | guinea pig | SC, SV, SL | mesenchymal cells in SV/ST | NO | Raphael, Y. 1996 [7]; Weiss, M.A. 1997 [15]; Ishimoto, S. 2002 [5] |
| AAV | mouse | IHC, OHC, SC, SG, SL, SV | IHC, OHC, SC, SG, SL, SV | YES | Iizuka, T. 2008 [12]; Shu, Y. et al., 2016 [16] |
| AAV | guinea pig | Not reported | spiral limbus, SL, SG, organ of Corti | Lalwani, K. 2000 [17] | |
| HSV | guinea pig | Not reported | fibrocytes (types I, II, IV), mesenchymal cells, HCs | NO | Derby, M.L. 1999 [8] |
| LV | guinea pig | Not reported | mesenchymal cells in ST | Han, J.J. 1999 [9] | |
| SEV | guinea pig | IHC, OHC, SC, | mesenchymal cells in ST | NO | Kanzaki, S. 2007 [10] |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanzaki, S. Gene Delivery into the Inner Ear and Its Clinical Implications for Hearing and Balance. Molecules 2018, 23, 2507. https://doi.org/10.3390/molecules23102507
Kanzaki S. Gene Delivery into the Inner Ear and Its Clinical Implications for Hearing and Balance. Molecules. 2018; 23(10):2507. https://doi.org/10.3390/molecules23102507
Chicago/Turabian StyleKanzaki, Sho. 2018. "Gene Delivery into the Inner Ear and Its Clinical Implications for Hearing and Balance" Molecules 23, no. 10: 2507. https://doi.org/10.3390/molecules23102507
APA StyleKanzaki, S. (2018). Gene Delivery into the Inner Ear and Its Clinical Implications for Hearing and Balance. Molecules, 23(10), 2507. https://doi.org/10.3390/molecules23102507