Quercetagetin and Patuletin: Antiproliferative, Necrotic and Apoptotic Activity in Tumor Cell Lines
Abstract
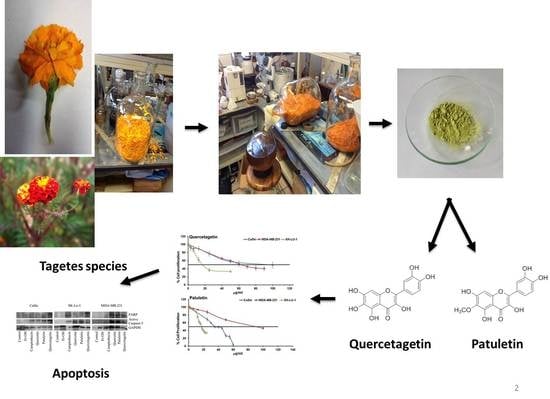
:1. Introduction
2. Results
2.1. Identification of Compounds 2 and 3
2.2. Antiproliferative Activity
2.3. Necrotic Activity in Tumor Cells
2.4. Apoptotic Bodies under DAPI Staining
Detection of Active Caspase-3, Active Caspase-8, Active Caspase-9 and PARP
2.5. Effect of Quercetin, Quercetagetin and Patuletin on Non-Tumor Cells
2.6. Necrotic Effect of Quercetin, Quercetagetin and Patuletin in Non-Tumor Cells
3. Discussion
4. Materials and Methods
4.1. Instrumentation
4.2. Plant Materials
4.3. Extraction and Isolation
4.4. Compound Characterization
4.5. Cell Culture
4.6. Cell Proliferation Assay
4.7. Determination of LDH
4.8. Observation of Morphological Characteristics of Apoptotic Cells by Fluorescence Microscopy
4.9. CFSE-Labeling Assay
4.10. Immunolocalization of Active Caspase-3, Active Caspase 8 and Active Caspase-9 by Flow Cytometry
4.11. Western Blot Analysis
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Castro, A.J.; Villarreal, M.L.; Salazar-Olivo, L.A.; Gomez-Sanchez, M.; Dominguez, F.; Garcia-Carranca, A. Mexican medicinal plants used for cancer treatment: Pharmacological, phytochemical and ethnobotanical studies. J. Ethnopharmacol. 2011, 133, 945–972. [Google Scholar] [CrossRef] [PubMed]
- Neher, R.T. The ethnobotany of Tagetes. Econ. Bot. 1968, 22, 317–325. [Google Scholar] [CrossRef]
- Vasudevan, P.; Kashyap, S.; Sharma, S. Tagetes: A multipurpose plant. Bioresour. Technol. 1997, 62, 29–35. [Google Scholar] [CrossRef]
- Martínez, R.; Diaz, B.; Vásquez, L.; Compagnone, R.S.; Tillett, S.; Canelón, D.J.; Torrico, F.; Suárez, A.I. Chemical composition of essential oils and toxicological evaluation of Tagetes erecta and Tagetes patula from Venezuela. J. Essent. Oil Bear. Plants 2009, 12, 476–481. [Google Scholar] [CrossRef]
- Priyanka, D.; Shalini, T.; Navneet, V.K. A brief study on Marigold (Tagetes species): A review. Int. Res. J. Pharm. 2013, 4, 43–48. [Google Scholar]
- Devika, R.; Justin Koilpillai, Y. Phytochemical screening studies of bioactive compounds of Tagetes erecta. Int. J. Pharm. BioSci. 2012, 3, 596–602. [Google Scholar]
- Xu, L.; Chen, J.; Qi, H.; Shi, Y. Phytochemicals and their biological activities of plants in Tagetes L. Chin. Herb. Med. 2012, 4, 103–117. [Google Scholar] [CrossRef]
- Wang, T.; Li, Q.; Bi, K. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef]
- Singh, P.; Krishna, A.; Kumar, V.; Krishna, S.; Singh, K.; Gupta, M.; Singh, S. Chemistry and biology of industrial crop Tagetes species: A review. J. Essent. Oil Res. 2016, 28, 1–14. [Google Scholar] [CrossRef]
- Abdel-Wahhab, M.A.; Said, A.; Huefner, A. NMR and radical scavenging activities of patuletin from Urtica urens. Against aflatoxin B1. Pharm. Biol. 2005, 43, 515–525. [Google Scholar] [CrossRef]
- Gong, Y.; Liu, X.; He, W.-H.; Xu, H.-G.; Yuan, F.; Gao, Y.-X. Investigation into the antioxidant activity and chemical composition of alcoholic extracts from defatted marigold (Tagetes erecta L.) residue. Fitoterapia 2012, 83, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Venegas, G.; Jiménez-Estrada, M.; Maldonado, S. The effect of flavonoids on transduction mechanisms in lipopolysaccharide-treated human gingival fibroblasts. Int. Immunopharmacol. 2007, 7, 1199–1210. [Google Scholar] [CrossRef] [PubMed]
- Tu, B.; Liu, Z.-J.; Chen, Z.-F.; Ouyang, Y.; Hu, Y.-J. Understanding the structure–activity relationship between quercetin and naringenin: In vitro. RSC Adv. 2015, 5, 106171–106181. [Google Scholar] [CrossRef]
- Flavonoids: Recent Advances as Anticancer Drugs. Available online: http://www.eurekaselect.com/85957/article (accessed on 16 August 2018).
- Flavonoids in Health and Disease. Available online: https://www.crcpress.com/Flavonoids-in-Health-and-Disease-Second-Edition/Rice-Evans-Packer/p/book/9780824742348 (accessed on 27 September 2018).
- Aherne, S.A.; O’Brien, N.M. Dietary flavonols: Chemistry, food content, and metabolism. Nutrition 2002, 18, 75–81. [Google Scholar] [CrossRef]
- Ren, W.; Qiao, Z.; Wang, H.; Zhu, L.; Zhang, L. Flavonoids: Promising anticancer agents. Med. Res. Rev. 2003, 23, 519–534. [Google Scholar] [CrossRef] [PubMed]
- López-Lázaro, M. Flavonoids as anticancer agents: Structure-activity relationship study. Curr. Med. Chem. Anticancer Agents 2002, 2, 691–714. [Google Scholar] [CrossRef] [PubMed]
- Ravishankar, D.; Rajora, A.K.; Greco, F.; Osborn, H.M.I. Flavonoids as prospective compounds for anti-cancer therapy. Int. J. Biochem. Cell Biol. 2013, 45, 2821–2831. [Google Scholar] [CrossRef] [PubMed]
- Chidambara Murthy, K.N.; Kim, J.; Vikram, A.; Patil, B.S. Differential inhibition of human colon cancer cells by structurally similar flavonoids of citrus. Food Chem. 2012, 132, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Kothandan, G.; Gadhe, C.G.; Madhavan, T.; Choi, C.H.; Cho, S.J. Docking and 3D-QSAR (quantitative structure activity relationship) studies of flavones, the potent inhibitors of p-glycoprotein targeting the nucleotide binding domain. Eur. J. Med. Chem. 2011, 46, 4078–4088. [Google Scholar] [CrossRef] [PubMed]
- Fink, S.L.; Cookson, B.T. Apoptosis, pyroptosis, and necrosis: Mechanistic description of dead and dying eukaryotic cells. Infect. Immun. 2005, 73, 1907–1916. [Google Scholar] [CrossRef] [PubMed]
- Amrutha, K.; Nanjan, P.; Shaji, S.K.; Sunilkumar, D.; Subhalakshmi, K.; Rajakrishna, L.; Banerji, A. Discovery of lesser known flavones as inhibitors of NF-κB signaling in MDA-MB-231 breast cancer cells—A SAR study. Bioorg. Med. Chem. Lett. 2014, 24, 4735–4742. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Liu, Y.; Wang, M.; Qian, Y.; Dong, X.; Gu, H.; Wang, H.; Guo, S.; Hisamitsu, T. Quercetin-induced apoptosis of HT-29 colon cancer cells via inhibition of the Akt-CSN6-Myc signaling axis. Mol. Med. Rep. 2016, 14, 4559–4566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maurya, A.K.; Vinayak, M. Anticarcinogenic action of quercetin by downregulation of phosphatidylinositol 3-kinase (PI3K) and protein kinase C (PKC) via induction of p53 in hepatocellular carcinoma (HepG2) cell line. Mol. Med. Rep. 2015, 42, 1419–1429. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Somasagara, R.R.; Hegde, M.; Nishana, M.; Tadi, S.K.; Srivastava, M.; Choudhary, B.; Raghavan, S.C. Quercetin, a natural flavonoid interacts with DNA, arrests cell cycle and causes tumor regression by activating mitochondrial pathway of apoptosis. Sci. Rep. 2016, 6, 24049. [Google Scholar] [CrossRef] [PubMed]
- Chemistry and Biological Activities of Flavonoids: An Overview. Available online: https://www.hindawi.com/journals/tswj/2013/162750/ (accessed on 17 August 2018).
- Bate-Smith, E.C. Quercetagetin and patuletin in Eriocaulon. Phytochemistry 1969, 8, 1035–1037. [Google Scholar] [CrossRef]
- Kaisoon, O.; Konczak, I.; Siriamornpun, S. Potential health enhancing properties of edible flowers from Thailand. Food Res. Int. 2012, 46, 563–571. [Google Scholar] [CrossRef]
- Vallisuta, O.; Nukoolkarn, V.; Mitrevej, A.; Sarisuta, N.; Leelapornpisid, P.; Phrutivorapongkul, A.; Sinchaipanid, N. In vitro studies on the cytotoxicity, and elastase and tyrosinase inhibitory activities of marigold (Tagetes erecta L.) flower extracts. Exp. Ther. Med. 2014, 7, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Lv, C.; Wang, J.; Gao, Q.; Zhu, H.; Wen, H. Patuletin induces apoptosis of human breast cancer SK-BR-3 cell line via inhibiting fatty acid synthase gene expression and activity. Oncol. Lett. 2017, 14, 7449–7454. [Google Scholar] [CrossRef] [PubMed]
- Holder, S.; Zemskova, M.; Zhang, C.; Tabrizizad, M.; Bremer, R.; Neidigh, J.W.; Lilly, M.B. Characterization of a potent and selective small-molecule inhibitor of the PIM1 kinase. Mol. Cancer Ther. 2007, 6, 163–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, G.-J.; Han, S.-C.; Ock, J.-W.; Kang, H.-K.; Yoo, E.-S. Anti-inflammatory effect of quercetagetin, an active component of immature citrus unshiu, in HaCaT human keratinocytes. Biomol. Ther. 2013, 21, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Kobayakawa, J.; Sato-Nishimori, F.; Moriyasu, M.; Matsukawa, Y. G2-M arrest and antimitotic activity mediated by casticin, a flavonoid isolated from Viticis Fructus (Vitex rotundifolia Linne fil.). Cancer Lett. 2004, 208, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Lee, H.-B.; Jung, G.O.; Oh, J.T.; Park, D.E.; Chae, K.M. Effect of quercetin on apoptosis of PANC-1 cells. J. Korean Surg. Soc. 2013, 85, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.-W.; Zhang, X.-J.; Lin, K.-Y.; Ye, H.; Feng, S.-Y.; Zhang, H.; Chen, Y.-Q. Camptothecin induces apoptosis in cancer cells via MicroRNA-125b-mediated mitochondrial pathways. Mol. Pharmacol. 2012, 81, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Mazhar, M.; Faizi, S.; Gul, A.; Kabir, N.; Simjee, S.U. Effects of naturally occurring flavonoids on ferroportin expression in the spleen in iron deficiency anemia in vivo. RSC Adv. 2017, 7, 23238–23245. [Google Scholar] [CrossRef] [Green Version]
- Periasamy, R.; Kalal, I.G.; Krishnaswamy, R.; Viswanadha, V. Quercetin protects human peripheral blood mononuclear cells from OTA-induced oxidative stress, genotoxicity, and inflammation. Environ. Toxicol. 2016, 31, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Kueng, W.; Silber, E.; Eppenberger, U. Quantification of cells cultured on 96-well plates. Anal. Biochem. 1989, 182, 16–19. [Google Scholar] [CrossRef]
- Fotakis, G.; Timbrell, J.A. In vitro cytotoxicity assays: Comparison of LDH, neutral red, MTT and protein assay in hepatoma cell lines following exposure to cadmium chloride. Toxicol. Lett. 2006, 160, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Quah, B.J.C.; Parish, C.R. The use of carboxyfluorescein diacetate succinimidyl ester (CFSE) to monitor lymphocyte proliferation. J. Vis. Exp. 2010, 2259. [Google Scholar] [CrossRef] [PubMed]
- Tuschl, H.; Schwab, C.E. Flow cytometric methods used as screening tests for basal toxicity of chemicals. Toxicol. In Vitro 2004, 18, 483–491. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds quercetin, quercetagetin and patuletin are available from the authors. |













| 2 a | 3 | ||||||
|---|---|---|---|---|---|---|---|
| Measured b | Literature c | ||||||
| Position | δc (J in Hz) | δH (J in Hz) | Position | δc (J in Hz) | δH (J in Hz) | δc (J in Hz) | δH (J in Hz) |
| 4 | 175.84 | 4 | 177.58 | 177.86 | |||
| 5 | 153.58 | 9 | 158.46 d | 158.76 d | |||
| 7 | 148.81 | 5 | 153.61 d | 153.89 d | |||
| 4′ | 147.57 | 7 | 153.00 d | 153.28 d | |||
| 2 | 146.64 | 4′ | 148.82 | 149.10 | |||
| 6 | 145.88 | 2 | 148.28 | 148.56 | |||
| 3′ | 145.04 | 3′ | 146.22 | 146.51 | |||
| 3 | 135.32 | 3 | 136.96 | 137.25 | |||
| 9 | 128.53 | 6 | 132.17 | 132.47 | |||
| 1′ | 122.21 | 1′ | 124.14 | 124.43 | |||
| 6′ | 119.91 | 7.53 (dd, J = 8.5, 2.2) | 6′ | 121.72 | 7.64 dd (J = 8.5, 2.2) | 122.02 | 7.65 dd (J = 8.4, 2.2) |
| 5′ | 115.59 | 6.88 (d, J = 8.5) | 2′ | 116.22 | 7.74 d (J = 2.2) | 116.52 | 7.76 (J = 2.2) |
| 2′ | 115.03 | 7.66 (d, J = 2.2) | 5′ | 116.02 | 6.89 d (J = 8.5) | 116.31 | 6.92 d (J = 8.5) |
| 10 | 103.31 | 10 | 104.95 | 105.25 | |||
| 8 | 93.22 | 6.49 (s) | 8 | 94.70 | 6.50 s | 95.00 | 6.52 s |
| OCH3 | 60.97 | 3.89 s | 61.27 | 3.92 s | |||
| C5-OH | 12.25 (s) | ||||||
| C6-OH | 10.48 (s) | ||||||
| C3′-OH | 9.55 (s) | ||||||
| C4′-OH | 9.28 (s) | ||||||
| C3-OH | 9.19 (s) | ||||||
| C7-OH | 8.65 (s) | ||||||
| Compound/Cell line | CaSki | MDA-MB-231 | SK-LU-1 |
|---|---|---|---|
| Quercetin | 39 (129) | 55 (129) | 39 (181) |
| Quercetagetin | 88 (276) | 60 (188) | 19 (59) |
| Patuletin | 37 (111) | 86 (258) | 18 (54.17) |
| CaSki | MDA-MB-231 | SK-LU-1 | |
|---|---|---|---|
| Quercetin | |||
| Control | 4 | 3 | 4 |
| EtOH | 5 | 3 | 5 |
| Quercetin | 14 | 37 | 45 |
| Camptothecin | 30 | 35 | 60 |
| Quercetagetin | |||
| Control | 4 | 4 | 4 |
| EtOH | 6 | 5 | 5 |
| Quercetagetin | 31 | 20 | 45 |
| Camptothecin | 50 | 25 | 50 |
| Patuletin | |||
| Control | 3 | 5 | 3 |
| EtOH | 4 | 5 | 5 |
| Patuletin | 40 | 43 | 36 |
| Camptothecin | 65 | 32 | 43 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarado-Sansininea, J.J.; Sánchez-Sánchez, L.; López-Muñoz, H.; Escobar, M.L.; Flores-Guzmán, F.; Tavera-Hernández, R.; Jiménez-Estrada, M. Quercetagetin and Patuletin: Antiproliferative, Necrotic and Apoptotic Activity in Tumor Cell Lines. Molecules 2018, 23, 2579. https://doi.org/10.3390/molecules23102579
Alvarado-Sansininea JJ, Sánchez-Sánchez L, López-Muñoz H, Escobar ML, Flores-Guzmán F, Tavera-Hernández R, Jiménez-Estrada M. Quercetagetin and Patuletin: Antiproliferative, Necrotic and Apoptotic Activity in Tumor Cell Lines. Molecules. 2018; 23(10):2579. https://doi.org/10.3390/molecules23102579
Chicago/Turabian StyleAlvarado-Sansininea, Jesús J., Luis Sánchez-Sánchez, Hugo López-Muñoz, María L. Escobar, Fernando Flores-Guzmán, Rosario Tavera-Hernández, and Manuel Jiménez-Estrada. 2018. "Quercetagetin and Patuletin: Antiproliferative, Necrotic and Apoptotic Activity in Tumor Cell Lines" Molecules 23, no. 10: 2579. https://doi.org/10.3390/molecules23102579
APA StyleAlvarado-Sansininea, J. J., Sánchez-Sánchez, L., López-Muñoz, H., Escobar, M. L., Flores-Guzmán, F., Tavera-Hernández, R., & Jiménez-Estrada, M. (2018). Quercetagetin and Patuletin: Antiproliferative, Necrotic and Apoptotic Activity in Tumor Cell Lines. Molecules, 23(10), 2579. https://doi.org/10.3390/molecules23102579