Ultrasensitive Electrochemical Sensor Based on Polyelectrolyte Composite Film Decorated Glassy Carbon Electrode for Detection of Nitrite in Curing Food at Sub-Micromolar Level
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Surface Morphology
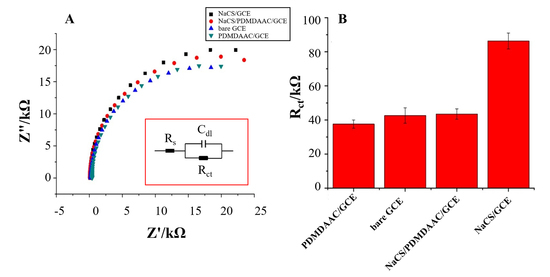
2.2. Electrochemical Characterization of NaCS/PDMDAAC/GCE
2.3. Electrochemical Behavior of Nitrite on NaCS/PDMDAAC/GCE
2.4. Optimization of Analytical Conditions
2.4.1. Effect of the Modifier Loading Amount
2.4.2. Effect of PDMDAAC Concentration
2.4.3. Effect of pH
2.4.4. Effect of Accumulation Time
2.5. Standard Curves, Linear Ranges, and Detection Limit
2.6. Selectivity, Reproducibility, and Stability of NaCS/PDMDAAC/GCE
2.7. Determination of Actual Samples
3. Conclusions
4. Materials and Methods
4.1. Chemicals
4.2. Fabrication of NaCS/PDMDAAC/GCE
4.3. Electrochemical Measurements
4.4. Pretreatment of Ham Sausage Samples
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Noor, N.S.; Tan, L.L.; Heng, L.Y.; Chong, K.F.; Tajuddin, S.N. Acrylic microspheres-based optosensor for visual detection of nitrite. Food Chem. 2016, 207, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Kumar, J.V.; Karthik, R.; Chen, S.M.; Balasubramanian, P.; Muthuraj, V.; Selvam, V. A novel cerium tungstate nanosheets modified electrode for the effective electrochemical detection of carcinogenic nitrite ions. Electroanalysis 2017, 29, 2385–2394. [Google Scholar] [CrossRef]
- Rastogi, P.; Ganesan, V.; Krishnamoorthi, S. A promising electrochemical sensing platform based on a silver nanoparticles decorated copolymer for sensitive nitrite determination. J. Mater. Chem. A 2013, 2, 933–943. [Google Scholar] [CrossRef]
- Manassaram, D.M.; Backer, L.C.; Moll, D.M. A review of nitrites in drinking water: Maternal exposure and adverse reproductive and developmental outcomes. Environ. Health Perspect. 2006, 114, 320–327. [Google Scholar] [CrossRef] [PubMed]
- Bukowski, J.; Somers, G.; Bryanton, J. Agricultural contamination of groundwater as a possible risk factor for growth restriction or prematurity. J. Occup. Environ. Med. 2001, 43, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Brender, J.D.; Olive, J.M.; Felkner, M.; Suarez, L.; Marckwardt, W.; Hendricks, K.A. Dietary nitrites and nitrites, nitrosatable drugs, and neural tube defects. Epidemiology 2004, 15, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Kikura-Hanajiri, R.; Martin, R.S.; Lunte, S.M. Indirect measurement of nitric oxide production by monitoring nitrite and nitrite using microchip electrophoresis with electrochemical detection. Anal. Chem. 2002, 74, 6370–6377. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guidelines for Drinking-Water Quality, 3rd ed.; World Health Organization: Geneva, Switzerland, 2008; ISBN 9789241547611. [Google Scholar]
- Mao, Y.; Bao, Y.; Han, D.; Zhao, B. Research progress on nitrite electrochemical sensor. Chin. J. Anal. Chem. 2018, 46, 147–156. [Google Scholar] [CrossRef]
- Li, Y.; Whitaker, J.S.; McCarty, C.L. Reversed-phase liquid chromatography/electrospray ionization/mass spectrometry with isotope dilution for the analysis of nitrite and nitrite in water. J. Chromatogr. 2011, 1218, 476–483. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Zhang, K.; Wang, C.; Luo, X.; Zhang, S. Effective indirect enrichment and determination of nitrite ion in water and biological samples using ionic liquid-dispersive liquid-liquid microextraction combined with high-performance liquid chromatography. J. Chromatogr. 2011, 1218, 3595–3600. [Google Scholar] [CrossRef] [PubMed]
- Manzoori, J.L.; Sorouraddin, M.H.; Hajishabani, A.M. Spectrophotometric determination of nitrite based on its catalytic effect on the oxidation of carminic acid by bromate. Talanta 1998, 46, 1379–1386. [Google Scholar] [CrossRef]
- Pourreza, N.; Fat’Hi, M.R.; Hatami, A. Indirect cloud point extraction and spectrophotometric determination of nitrite in water and meat products. Microchem. J. 2012, 104, 22–25. [Google Scholar] [CrossRef]
- Peng, Q.; Xie, M.G.; Koon-Gee, N.; Kang, E.T. A new nitrite-selective fluorescent sensor fabricated from surface-initiated atom-transfer radical polymerization. Chem. Lett. 2005, 34, 1628–1629. [Google Scholar] [CrossRef]
- Jiao, C.X.; Niu, C.G.; Huan, S.Y.; Shen, Q.; Yang, Y.; Shen, G.L.; Yu, R.Q. A reversible chemosensor for nitrite based on the fluorescence quenching of a carbazole derivative. Talanta 2004, 64, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Ma, J.; Yuan, D.; Feng, S.; Su, H.; Huang, Y.; Shangguan, Q. Sequential determination of multi-nutrient elements in natural water samples with a reverse flow injection system. Talanta 2017, 167, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Zhang, M.; Huang, Y.; Yuan, D.; Zhu, Y. Simultaneous determination of nanomolar nitrite and nitrite in seawater using reverse flow injection analysis coupled with a long path length liquid waveguide capillary cell. Talanta 2013, 117, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Ning, J.; He, Q.; Luo, X.; Wang, M.; Liu, D.; Wang, J.; Liu, J.; Li, G. Rapid and sensitive determination of vanillin based on a glassy carbon electrode modified with Cu2O-electrochemically reduced graphene oxide nanocomposite film. Sensors 2018, 18, 2762. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Liu, J.; Liang, J.; Liu, X.; Li, W.; Liu, Z.; Ding, Z.; Tuo, D. Towards Improvements for Penetrating the Blood–Brain Barrier—Recent Progress from a Material and Pharmaceutical Perspective. Cells 2018, 7, 24. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Deng, P.; Liang, J. Preparation of Cu2O-reduced graphene nanocomposite modified electrodes towards ultrasensitive dopamine detection. Sensors 2018, 18, 199. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Chen, D.; Deng, P.; Liang, J. Fabrication of amine-modified magnetite-electrochemically reduced graphene oxide nanocomposite modified glassy carbon electrode for sensitive dopamine determination. Nanomaterials 2018, 8, 194. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Liu, J.; Liang, J.; Liu, X.; Tuo, D.; Li, W. Chemically Surface Tunable Solubility Parameter for Controllable Drug Delivery—An Example and Perspective from Hollow PAA-Coated Magnetite Nanoparticles with R6G Model Drug. Materials 2018, 11, 247. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Liu, J.; Liu, X.; Li, G.; Deng, P.; Liang, J. Manganese dioxide Nanorods/electrochemically reduced graphene oxide nanocomposites modified electrodes for cost-effective and ultrasensitive detection of Amaranth. Colloid Surf. B 2018, 172, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Song, X.; Ge, X.; Zhang, H.; Wang, G.; Zhang, Y.; Zhao, H. In situ growth of α-Fe2O3 nanorod arrays on 3d carbon foam as an efficient binder-free electrode for highly sensitive and specific determination of nitrite. J. Mater. Chem. A 2017, 5, 4726–4736. [Google Scholar] [CrossRef]
- Mehmeti, E.; Stanković, D.M.; Hajrizi, A.; Kalcher, K. The use of graphene nanoribbons as efficient electrochemical sensing material for nitrite determination. Talanta 2016, 159, 34–39. [Google Scholar] [CrossRef] [PubMed]
- Rabti, A.; Aoun, S.B.; Raouafi, N. A sensitive nitrite sensor using an electrode consisting of reduced graphene oxide functionalized with ferrocene. Microchim. Acta 2016, 183, 3111–3117. [Google Scholar] [CrossRef]
- Dai, J.; Deng, D.; Yuan, Y.; Zhang, J.; Deng, F.; He, S. Amperometric nitrite sensor based on a glassy carbon electrode modified with multi-walled carbon nanotubes and poly(toluidine blue). Microchim. Acta 2016, 183, 1553–1561. [Google Scholar] [CrossRef]
- Shivakumar, M.; Nagashree, K.L.; Manjappa, S.; Dharmaprakash, M.S. Electrochemical detection of nitrite using glassy carbon electrode modified with silver nanospheres (AgNS) obtained by green synthesis using pre-hydrolysed liquor. Electroanalysis 2017, 29, 1434–1442. [Google Scholar] [CrossRef]
- Seo, Y.; Manivannan, S.; Kang, I.; Lee, S.W.; Kim, K. Gold dendrites co-deposited with m13 virus as a biosensor platform for nitrite ions. Biosens. Bioelectron. 2017, 94, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Kung, C.W.; Li, Y.S.; Lee, M.H.; Wang, S.Y.; Chiang, W.H.; Ho, K.C. In-situ growth of porphyrinic metal-organic framework nanocrystals on graphene nanoribbons for electrocatalytic oxidation of nitrite. J. Mater. Chem. A 2016, 4, 10673–10682. [Google Scholar] [CrossRef]
- Kung, C.W.; Chang, T.H.; Chou, L.Y.; Hupp, J.T.; Farha, O.K.; Ho, K.C. Porphyrin-based metal–organic framework thin films for electrochemical nitrite detection. Electrochem. Commun. 2015, 58, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.; Liang, S.; Fan, J.; Sheng, G.; Luo, X. Amperometric sensing of nitrite using a glassy carbon electrode modified with a multilayer consisting of carboxylated nanocrystalline cellulose and poly(diallyldimethyl ammonium) ions in a pedot host. Microchim. Acta 2016, 183, 2031–2037. [Google Scholar] [CrossRef]
- Chen, L.; Liu, X.; Wang, C.; Lv, S.; Chen, C. Amperometric nitrite sensor based on a glassy carbon electrode modified with electrodeposited poly(3,4-ethylenedioxythiophene) doped with a polyacenic semiconductor. Microchim. Acta 2017, 184, 2073–2079. [Google Scholar] [CrossRef]
- Masumi, T.; Matsushita, Y.; Dan, A.; Takama, R.; Saito, K.; Kuroda, K.; Fukushima, K. Adsorption behavior of poly(dimethyl-diallylammonium chloride) on pulp fiber studied by cryo-time-of-flight secondary ion mass spectrometry and cryo-scanning electron microscopy. Appl. Surf. Sci. 2014, 289, 155–159. [Google Scholar] [CrossRef]
- Wang, B.; Okoth, O.K.; Yan, K.; Zhang, J. A highly selective electrochemical sensor for 4-chlorophenol determination based on molecularly imprinted polymer and PDDA-functionalized graphene. Sens. Actuators B Chem. 2016, 236, 294–303. [Google Scholar] [CrossRef]
- Yang, J.; Lin, Q.; Yin, W.; Jiang, T.; Zhao, D.; Jiang, L. A novel nonenzymatic glucose sensor based on functionalized pdda-graphene/cuo nanocomposites. Sens. Actuators B Chem. 2017, 253, 1087–1095. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, W.; Du, M.; Jiao, K. A PDDA/poly(2,6-pyridinedicarboxylic acid)-CNTs composite film DNA electrochemical sensor and its application for the detection of specific sequences related to PAT gene and NOS gene. Talanta 2008, 75, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Yao, S.J.; Guan, Y.X.; Lin, D.Q. Preparation and characterization of NaCS-cmc/PDMDAAC capsules. Colloids Surf. B Biointerfaces 2005, 45, 136–143. [Google Scholar] [CrossRef] [PubMed]
- Zeng, X.H.; Danquah, M.K.; Zheng, C.; Potumarthi, R.; Chen, X.D.; Lu, Y.H. NaCS-PDMDAAC immobilized autotrophic cultivation of chlorella sp. For wastewater nitrogen and phosphate removal. Chem. Eng. J. 2004, 99, 185–192. [Google Scholar] [CrossRef]
- Zhang, J.; Guan, Y.X.; Ji, Z.; Yao, S.J. Effects on membrane properties of NaCS–PDMDAAC capsules by adding inorganic salts. J. Membr. Sci. 2006, 277, 270–276. [Google Scholar] [CrossRef]
- Wang, M.; Chen, K.; Liu, J.; He, Q.; Li, G.; Li, F. Efficiently enhancing electrocatalytic activity of α-MnO2 nanorods/N-doped ketjenblack carbon for oxygen reduction reaction and oxygen evolution reaction using facile regulated hydrothermal treatment. Catalysts 2018, 8, 138. [Google Scholar] [CrossRef]
- Chen, K.; Wang, M.; Li, G.; He, Q.; Liu, J.; Li, F. Spherical α-MnO2 supported on N-KB as efficient electrocatalyst for oxygen reduction in al–air battery. Materials 2018, 11, 601. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Wang, S.; Duan, Y.Y. Towards gel-free electrodes: A systematic study of electrode-skin impedance. Sens. Actuators B Chem. 2017, 241, 1244–1255. [Google Scholar] [CrossRef]
- Li, G.; Zhang, D.; Wang, S.; Duan, Y.Y. Novel passive ceramic based semi-dry electrodes for recording electroencephalography signals from the hairy scalp. Sens. Actuators B Chem. 2016, 237, 167–178. [Google Scholar] [CrossRef]
- Bai, W.; Sheng, Q.; Zheng, J. Hydrophobic interface controlled electrochemical sensing of nitrite based on one step synthesis of polyhedral oligomeric silsesquioxane/reduced graphene oxide nanocomposite. Talanta 2016, 150, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, F.; Chen, Z. An electropolymerized nile blue sensing film-based nitrite sensor and application in food analysis. Anal. Chim. Acta 2008, 623, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.J.; Zhang, P.P.; Wang, A.J.; Zhang, Y.; Dong, W.J.; Chen, J.R. One-pot hydrothermal synthesis of uniform β-MnO2 nanorods for nitrite sensing. J. Colloid Interface Sci. 2011, 359, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Ghanei-Motlagh, M.; Taher, M.A. A novel electrochemical sensor based on silver/halloysite nanotube/molybdenum disulfide nanocomposite for efficient nitrite sensing. Biosens. Bioelectron. 2018, 109, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Haldorai, Y.; Hwang, S.K.; Gopalan, A.I.; Huh, Y.S.; Han, Y.K.; Voit, W.; Saianand, G.; Lee, K.P. Direct electrochemistry of cytochrome c immobilized on titanium nitride/multi-walled carbon nanotube composite for amperometric nitrite biosensor. Biosens. Bioelectron. 2016, 79, 543–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranjani, B.; Kalaiyarasi, J.; Pavithra, L.; Devasena, T.; Pandian, K.; Gopinath, S. Amperometric determination of nitrite using natural fibers as template for titanium dioxide nanotubes with immobilized hemin as electron transfer mediator. Microchim. Acta 2018, 185, 194. [Google Scholar] [CrossRef] [PubMed]









| Electrodes | Methods | Linear Ranges (μmol·L–1) | LOD (μmol·L–1) | References |
|---|---|---|---|---|
| POSS/rGO/GCE | CA | 0.5–120 | 0.08 | [45] |
| PNB/GCE | DPV | 0.5–100 | 0.1 | [46] |
| β-MnO2 NRs/GCE | CA | 0.29–26,090 | 0.29 | [47] |
| Ag/HNT/MoS2/CPE | CA | 2–425 | 0.7 | [48] |
| MWCNTs-TiN/GCE | CA | 1–2000 | 0.0014 | [49] |
| hemin/TNT/GCE | CA | 0.6–130 | 0.084 | [50] |
| NaCS/PDMDAAC/GCE | DPV | 150–0.04 | 0.043 | This work |
| No. | Current Response (μA) | |
|---|---|---|
| 8.0 × 10−5 mol⋅L–1 | 8 × 10−6 mol⋅L–1 | |
| 1 | 0.896 | 0.115 |
| 2 | 0.874 | 0.123 |
| 3 | 0.887 | 0.112 |
| 4 | 0.888 | 0.121 |
| 5 | 0.878 | 0.114 |
| 6 | 0.890 | 0.117 |
| 7 | 0.898 | 0.112 |
| 8 | 0.894 | 0.129 |
| 9 | 0.903 | 0.122 |
| Average | 0.889 | 0.118 |
| SD | 0.009 | 0.006 |
| RSD (%) | 1.05% | 4.89% |
| Samples | Content (mg/kg) | Added (mg/kg) | Founded (mg/kg) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| 1 | 27.32 | 30 | 58.52 | 104.0 | 2.3 |
| 2 | 26.87 | 30 | 57.32 | 102.6 | 1.4 |
| 3 | 26.24 | 30 | 55.71 | 98.2 | 3.3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ning, J.; Luo, X.; Wang, M.; Li, J.; Liu, D.; Rong, H.; Chen, D.; Wang, J. Ultrasensitive Electrochemical Sensor Based on Polyelectrolyte Composite Film Decorated Glassy Carbon Electrode for Detection of Nitrite in Curing Food at Sub-Micromolar Level. Molecules 2018, 23, 2580. https://doi.org/10.3390/molecules23102580
Ning J, Luo X, Wang M, Li J, Liu D, Rong H, Chen D, Wang J. Ultrasensitive Electrochemical Sensor Based on Polyelectrolyte Composite Film Decorated Glassy Carbon Electrode for Detection of Nitrite in Curing Food at Sub-Micromolar Level. Molecules. 2018; 23(10):2580. https://doi.org/10.3390/molecules23102580
Chicago/Turabian StyleNing, Jingheng, Xin Luo, Min Wang, Jiaojiao Li, Donglin Liu, Hou Rong, Donger Chen, and Jianhui Wang. 2018. "Ultrasensitive Electrochemical Sensor Based on Polyelectrolyte Composite Film Decorated Glassy Carbon Electrode for Detection of Nitrite in Curing Food at Sub-Micromolar Level" Molecules 23, no. 10: 2580. https://doi.org/10.3390/molecules23102580
APA StyleNing, J., Luo, X., Wang, M., Li, J., Liu, D., Rong, H., Chen, D., & Wang, J. (2018). Ultrasensitive Electrochemical Sensor Based on Polyelectrolyte Composite Film Decorated Glassy Carbon Electrode for Detection of Nitrite in Curing Food at Sub-Micromolar Level. Molecules, 23(10), 2580. https://doi.org/10.3390/molecules23102580