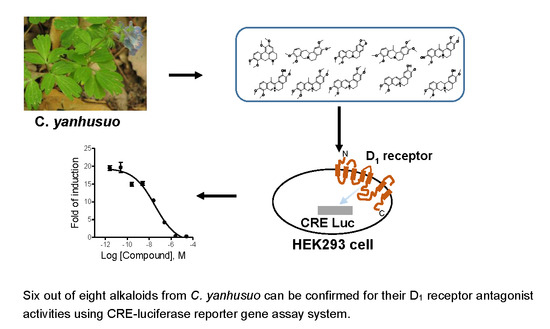
Identification of Alkaloids from Corydalis yanhusuo W. T. Wang as Dopamine D1 Receptor Antagonists by Using CRE-Luciferase Reporter Gene Assay
Abstract
:1. Introduction
2. Results
2.1. Development of Reporter Gene Assay for D1 Receptor Activation
2.2. Amenable for High-Throughput Screening (HTS)
2.3. Screening of D1 Dopamine Receptor Antagonists with Luciferase Reporter Gene Assay
2.4. Fluorescent Ca2+ Mobilization Assay
3. Discussion and Conclusions
4. Materials and Methods
4.1. Materials
4.2. Plant
4.3. Creation of the Stable Cell Line
4.4. Luciferase Reporter Gene Assay
4.5. Fluorescent Ca2+ Mobilization Assay
4.6. Data Processing
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vallone, D.; Picetti, R.; Borrelli, E. Structure and function of dopamine receptors. Neurosci. Biobehav. Rev. 2000, 24, 125–132. [Google Scholar] [CrossRef]
- Missale, C.; Nash, S.R.; Robinson, S.W.; Jaber, M.; Caron, M.G. Dopamine receptors: From structure to function. Physiol. Rev. 1998, 78, 189–225. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, J.M.; Gainetdinov, R.R. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol. Rev. 2011, 63, 182–217. [Google Scholar] [CrossRef] [PubMed]
- Beaulieu, J.M.; Espinoza, S.; Gainetdinov, R.R. Dopamine receptors—Iuphar review 13. Br. J. Pharmacol. 2015, 172, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Le Foll, B.; Gallo, A.; Le Strat, Y.; Lu, L.; Gorwood, P. Genetics of dopamine receptors and drug addiction: A comprehensive review. Behav. Pharmacol. 2009, 20, 1–17. [Google Scholar] [CrossRef] [PubMed]
- George, S.E.; Bungay, P.J.; Naylor, L.H. Functional analysis of the d2l dopamine receptor expressed in a camp-responsive luciferase reporter cell line. Biochem. Pharmacol. 1998, 56, 25–30. [Google Scholar] [CrossRef]
- Menon, V.; Ranganathn, A.; Jorgensen, V.H.; Sabio, M.; Christoffersen, C.T.; Uberti, M.A.; Jones, K.A.; Babu, P.S. Development of an aequorin luminescence calcium assay for high-throughput screening using a plate reader, the lumilux. Assay Drug Dev. Technol. 2008, 6, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Eglen, R.M.; Bosse, R.; Reisine, T. Emerging concepts of guanine nucleotide-binding protein-coupled receptor (gpcr) function and implications for high throughput screening. Assay Drug Dev. Technol. 2007, 5, 425–451. [Google Scholar] [CrossRef] [PubMed]
- Siehler, S. Cell-based assays in gpcr drug discovery. Biotechnol. J. 2008, 3, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Garvin, D.; Paguio, A.; Stecha, P.; Wood, K.; Fan, F. Luciferase reporter assay system for deciphering gpcr pathways. Curr. Chem. Genom. 2010, 4, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Hill, S.J.; Baker, J.G.; Rees, S. Reporter-gene systems for the study of g-protein-coupled receptors. Curr. Opin. Pharmacol. 2001, 1, 526–532. [Google Scholar] [CrossRef]
- Wang, J.B.; Mantsch, J.R. L-tetrahydropalamatine: A potential new medication for the treatment of cocaine addiction. Future Med. Chem. 2012, 4, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhang, Y.; Wang, Z.; Gong, N.; Kweon, T.D.; Vo, B.; Wang, C.; Zhang, X.; Chung, J.Y.; Alachkar, A.; et al. The antinociceptive properties of the corydalis yanhusuo extract. PLoS ONE 2016, 11, e0162875. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, C.; Wang, L.; Parks, G.S.; Zhang, X.; Guo, Z.; Ke, Y.; Li, K.W.; Kim, M.K.; Vo, B.; et al. A novel analgesic isolated from a traditional chinese medicine. Curr. Biol. 2014, 24, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Himmler, A.; Stratowa, C.; Czernilofsky, A.P. Functional testing of human dopamine d1 and d5 receptors expressed in stable camp-responsive luciferase reporter cell lines. J. Recept. Res. 1993, 13, 79–94. [Google Scholar] [CrossRef] [PubMed]
- Sunahara, R.K.; Guan, H.C.; O’Dowd, B.F.; Seeman, P.; Laurier, L.G.; Ng, G.; George, S.R.; Torchia, J.; Van Tol, H.H.; Niznik, H.B. Cloning of the gene for a human dopamine d5 receptor with higher affinity for dopamine than d1. Nature 1991, 350, 614–619. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Walker, G.B.; Zorn, S.H.; Wong, E.H. Asenapine: A novel psychopharmacologic agent with a unique human receptor signature. J. Psychopharmacol. 2009, 23, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Zhang, H.Q.; Liu, X.Y.; Hua, S.N.; Zhou, L.B.; Yu, J.; Tan, X.H. Identification of human dopamine receptors agonists from chinese herbs. Acta Pharmacol. Sin. 2007, 28, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.L.; Sealfon, S.C.; Gonzalez-Maeso, J. Group ii metabotropic glutamate receptors and schizophrenia. Cell. Mol. Life Sci. 2009, 66, 3777–3785. [Google Scholar] [CrossRef] [PubMed]
- Lappano, R.; Maggiolini, M. G protein-coupled receptors: Novel targets for drug discovery in cancer. Nat. Rev. Drug Discov. 2011, 10, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Yang, D.; Han, Y.; Wang, W.; Wang, N.; Yang, J.; Zeng, C. Dopamine d1-like receptors suppress the proliferation of macrophages induced by ox-ldl. Cell. Physiol. Biochem. Int. J. Exp. Cell. Physiol. Biochem. Pharmacol. 2016, 38, 415–426. [Google Scholar] [CrossRef] [PubMed]
- Bezerra Maia, E.H.M.S.; Praciano, P.C.; Gurgel Alves, J.A.; Martins, W.P.; Araujo, E.J.; Kane, S.C.; da Silva Costa, F. D1. Renal interlobar vein impedance index (rivi) as a first trimester marker for prediction of hypertensive disorders of pregnancy. J. Matern.-Fetal Neonatal Med. 2016, 29, 16. [Google Scholar] [CrossRef]
- Jose, P.A.; Yang, Z.; Zeng, C.; Felder, R.A. The importance of the gastrorenal axis in the control of body sodium homeostasis. Exp. Physiol. 2016, 101, 465–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.Y.; Jin, G.Z. Supraspinal d2 receptor involved in antinociception induced by l-tetrahydropalmatine. Zhongguo Yao Li Xue Bao = Acta Pharmacol. Sin. 1999, 20, 715–719. [Google Scholar]
- Ma, Z.Z.; Xu, W.; Jensen, N.H.; Roth, B.L.; Liu-Chen, L.Y.; Lee, D.Y. Isoquinoline alkaloids isolated from corydalis yanhusuo and their binding affinities at the dopamine d1 receptor. Molecules 2008, 13, 2303–2312. [Google Scholar] [CrossRef] [PubMed]
- Branchini, B.R.; Southworth, T.L.; Khattak, N.F.; Michelini, E.; Roda, A. Red- and green-emitting firefly luciferase mutants for bioluminescent reporter applications. Anal. Biochem. 2005, 345, 140–148. [Google Scholar] [CrossRef] [PubMed]
- Rosenbaum, D.M.; Rasmussen, S.G.; Kobilka, B.K. The structure and function of g-protein-coupled receptors. Nature 2009, 459, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Fan, F.; Wood, K.V. Bioluminescent assays for high-throughput screening. Assay Drug Dev. Technol. 2007, 5, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.; Jin, G.; Friedman, E.; Zhen, X. Recent development in studies of tetrahydroprotoberberines: Mechanism in antinociception and drug addiction. Cell. Mol. Neurobiol. 2008, 28, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.Y.; Li, L.; Chu, S.S.; Sun, Q.; Ma, Z.L.; Gu, X.P. Antinociceptive effects of dehydrocorydaline in mouse models of inflammatory pain involve the opioid receptor and inflammatory cytokines. Sci. Rep. 2016, 6, 27129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuda, H.; Tokuoka, K.; Wu, J.; Shiomoto, H.; Kubo, M. Inhibitory effects of dehydrocorydaline isolated from corydalis tuber against type I–IV allergic models. Biol. Pharm. Bull. 1997, 20, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Sohn, E.J.; Yoon, S.W.; Kim, C.G.; Lee, S.; Kim, J.Y.; Baek, N.; Kim, S.H. Anti-metastatic effect of dehydrocorydaline on h1299 non-small cell lung carcinoma cells via inhibition of matrix metalloproteinases and b cell lymphoma 2. Phytother. Res. 2017, 31, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.Y.; Shi, Y.; Zheng, S.L.; Jin, W.; Sun, H. Two new protoberberine quaternary alkaloids from corydalis yanhusuo. J. Asian Nat. Prod. Res. 2008, 10, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jin, Y.; Liu, Y.; Xiao, Y.; Feng, J.; Xue, X.; Zhang, X.; Liang, X. Two-dimensional rplc-rplc system with different ph in two dimensions for separation of alkaloids from corydalis yanhusuo w. T. Wang. J. Sep. Sci. 2009, 32, 2084–2089. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Jin, Y.; Liu, Y.; Xiao, Y.; Feng, J.; Xue, X.; Zhang, X.; Liang, X. Purification of alkaloids from corydalis yanhusuo w. T. Wang using preparative 2-D HPLC. J. Sep. Sci. 2009, 32, 1401–1406. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Guo, Z.; Zhang, J.; Zeng, J.; Zhang, X.; Liang, X. High-performance purification of quaternary alkaloids from corydalis yanhusuo w. T. Wang using a new polar-copolymerized stationary phase. J. Sep. Sci. 2011, 34, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Tian, Q.; Li, J.; Xie, X.; Sun, M.; Sang, H.; Zhou, C.; An, T.; Hu, L.; Ye, R.D.; Wang, M.W. Stereospecific induction of nuclear factor-kappaB activation by isochamaejasmin. Mol. Pharmacol. 2005, 68, 1534–1542. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Wu, L.H.; Zhang, G.F.; Xu, F.; Zhang, S.; Zhang, X.; Sun, L.; Yu, Y.; Zhang, Y.; Ye, R.D. 4′-hydroxywogonin suppresses lipopolysaccharide-induced inflammatory responses in raw 264.7 macrophages and acute lung injury mice. PLoS ONE 2017, 12, e0181191. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, Z.; Cox, D.P.; Civelli, O. Study on the activation of the opioid receptors by a set of morphine derivatives in a well-defined assay system. Neurochem. Res. 2012, 37, 410–416. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |




| No. | Compounds (CAS No.) | IC50 * (μM) with 95% Confidence Intervals | % Maximal Inhibition Mean ± SD (N = 3) | ||
|---|---|---|---|---|---|
| Luciferase | FLIPR | Luciferase | FLIPR | ||
| 1 | Glaucine | / | 9.408 | / | 101.6 ± 0.8452 |
| (475-81-0) | (6.568–13.48) | ||||
| 2 | Tetrahydropalmatine | 0.6437 | 2.240 | 72.53 ± 3.9953 | 92.64 ± 1.7945 |
| (2934-97-6) | (0.4442–0.9327) | (1.060–4.736) | |||
| 3 | Canadine | / | / | / | / |
| (522-97-4) | |||||
| 4 | Corydaline | 2.457 | 3.360 | 83.49 ± 1.4462 | 90.32 ± 1.9817 |
| (518-69-4) | (1.681–3.591) | (1.626–6.945) | |||
| 5 | 13-methyldehydrocorydalmine | 10.32 | 3.079 | 85.97 ± 1.7628 | 77.04 ± 6.6410 |
| (1126743-67-6) | (6.862–15.53) | (1.109–8.550) | |||
| 6 | Dehydrocorybulbine | 0.6209 | 0.6123 | 97.52 ± 0.6264 | 94.38 ± 0.1939 |
| (59870-72-3) | (0.4753–0.8109) | (0.4268–0.8784) | |||
| 7 | Dehydrocorydaline | 1.292 | 1.527 | 98.23 ± 0.3301 | 90.61 ± 0.6541 |
| (30045-16-0) | (1.005–1.661) | (0.5213–4.473) | |||
| 8 | Palmatine | 7.082 | / | 93.86 ± 0.4367 | / |
| (3486-67-7) | (5.297–9.469) | ||||
| 9 | Columbamine | 1.210 | 3.404 | 97.50 ± 0.2181 | 86.43 ± 0.7188 |
| (3621-36-1) | (0.8992–1.629) | (1.951–5.939) | |||
| 10 | N-methyltetrahydrocolumbamine | 13.36 | / | 78.15 ± 1.783 | / |
| (47528-98-3) | (8.246–21.66) | ||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, L.; Zhang, W.; Qiu, X.; Wang, C.; Liu, Y.; Wang, Z.; Yu, Y.; Ye, R.D.; Zhang, Y. Identification of Alkaloids from Corydalis yanhusuo W. T. Wang as Dopamine D1 Receptor Antagonists by Using CRE-Luciferase Reporter Gene Assay. Molecules 2018, 23, 2585. https://doi.org/10.3390/molecules23102585
Wu L, Zhang W, Qiu X, Wang C, Liu Y, Wang Z, Yu Y, Ye RD, Zhang Y. Identification of Alkaloids from Corydalis yanhusuo W. T. Wang as Dopamine D1 Receptor Antagonists by Using CRE-Luciferase Reporter Gene Assay. Molecules. 2018; 23(10):2585. https://doi.org/10.3390/molecules23102585
Chicago/Turabian StyleWu, Lehao, Weiyue Zhang, Xin Qiu, Chaoran Wang, Yanfang Liu, Zhiwei Wang, Yang Yu, Richard D. Ye, and Yan Zhang. 2018. "Identification of Alkaloids from Corydalis yanhusuo W. T. Wang as Dopamine D1 Receptor Antagonists by Using CRE-Luciferase Reporter Gene Assay" Molecules 23, no. 10: 2585. https://doi.org/10.3390/molecules23102585
APA StyleWu, L., Zhang, W., Qiu, X., Wang, C., Liu, Y., Wang, Z., Yu, Y., Ye, R. D., & Zhang, Y. (2018). Identification of Alkaloids from Corydalis yanhusuo W. T. Wang as Dopamine D1 Receptor Antagonists by Using CRE-Luciferase Reporter Gene Assay. Molecules, 23(10), 2585. https://doi.org/10.3390/molecules23102585