Multi-Gram Synthesis of Enantiopure 1,5-Disubstituted Tetrazoles Via Ugi-Azide 3-Component Reaction
Abstract
:1. Introduction
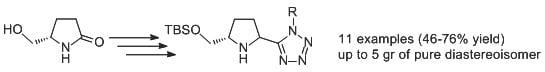
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Chemistry
3.2.1. Protection of Chiral Lactam 1
3.2.2. Reduction with Schwartz’s Reagent of Chiral Lactam 2 to Imine 3
3.2.3. UA-3CR of Imine 3 with TMS-N3 and R-NC
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Maleki, A.; Sarvary, A. Synthesis of tetrazoles via isocyanide-based reactions. RSC Adv. 2015, 5, 60938–60955. [Google Scholar] [CrossRef]
- Ostrovskii, V.A.; Trifonov, R.; Popova, E. Medicinal chemistry of tetrazoles. Russ. Chem. Bull. Int. Ed. 2012, 61, 768–780. [Google Scholar] [CrossRef]
- Ruijter, E.; Orru, R.V.A. Discovery of MCRs. In Multicomponent Reactions in Organic Synthesis; Zhu, J., Wang, Q., Wang, M.-X., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2014; pp. 13–38. [Google Scholar]
- Nielsen, T.E.; Schreiber, S.L. Towards the optimal screening collection: A synthesis strategy. Angew. Chem. Int. Ed. 2008, 47, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Waldmann, H. Synthesis of natural product inspired compound collections. Angew. Chem. Int. Ed. 2009, 48, 3224–3242. [Google Scholar] [CrossRef] [PubMed]
- Shmatova, O.I.; Nenajdenko, V.G. Tetrazole-Substituted five, six, and seven-membered cyclic amines bearing perfluoroalkyl groups—Efficient synthesis by Azido-Ugi reaction. Eur. J. Org. Chem. 2013, 2013, 6397–6403. [Google Scholar] [CrossRef]
- Zarezin, D.P.; Khrustalev, V.N.; Nenajdenko, V.G. Diastereoselectivity of Azido-Ugi reaction with secondary amines. Stereoselective synthesis of tetrazole derivatives. J. Org. Chem. 2017, 82, 6100–6107. [Google Scholar] [CrossRef] [PubMed]
- Banfi, L.; Basso, A.; Guanti, G.; Merlo, S.; Repetto, C.; Riva, R. A convergent synthesis of enantiopure bicyclic scaffolds through multicomponent Ugi reaction. Tetrahedron 2008, 64, 1114–1134. [Google Scholar] [CrossRef]
- Zhu, D.; Xia, L.; Pan, L.; Li, S.; Chen, R.; Mou, Y.; Chen, X. An asymmetric Ugi three-component reaction induced by chiral cyclic imines: Synthesis of morpholin– or piperazine–keto-carboxamide derivatives. J. Org. Chem. 2012, 77, 1386–1395. [Google Scholar] [CrossRef] [PubMed]
- Katsuyama, A.; Matsuda, A.; Ichikawa, S. Revisited mechanistic implications of the Joullié–Ugi three-component reaction. Org. Lett. 2016, 18, 2552–2555. [Google Scholar] [CrossRef] [PubMed]
- Moni, L.; De Moliner, F.; Garbarino, S.; Saupe, J.; Mang, C.; Basso, A. Exploitation of the Ugi 5-Center-4-Component Reaction (U-5C-4CR) for the generation of diverse libraries of polycyclic (spiro) compounds. Front. Chem. 2018, 6, 369. [Google Scholar] [CrossRef] [PubMed]
- Speich, E.; Banfi, L.; Moni, L.; Riva, R.; Rocca, V.; Basso, A. Zr-mediated synthesis of chiral cyclic imines and their application in Betti reactions. Chem. Heterocycl. Comp. 2018, 54, 329–333. [Google Scholar] [CrossRef]
- Szcześniak, P.; Maziarz, E.; Stecko, S.; Furman, M. Synthesis of polyhydroxylated piperidine and pyrrolidine peptidomimetics via one-pot sequential lactam reduction/Joullié–Ugi reaction. J. Org. Chem. 2015, 80, 3621–3633. [Google Scholar] [CrossRef] [PubMed]
- Oda, Y.; Sato, T.; Chida, N. Direct chemoselective allylation of inert amide carbonyls. Org. Lett. 2012, 14, 950–953. [Google Scholar] [CrossRef] [PubMed]
- Schedler, D.J.A.; Godfrey, A.G.; Ganem, B. Reductive deoxygenation by Cp2ZrHCl: Selective formation of imines via zirconation/hydrozirconation of amides. Tetrahedron Lett. 1993, 34, 5035–5038. [Google Scholar] [CrossRef]
- Schedler, D.J.A.; Li, J.; Ganem, B. Reduction of secondary carboxamides to imines. J. Org. Chem. 1996, 61, 4115–4119. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Kurpiewska, K.; Kalinowska-Tłuścik, J.; Herdtweck, E.; Dömling, A. α-Amino acid-isosteric α-amino tetrazoles. Chem. Eur. J. 2016, 22, 3009–3018. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 4a–k are available from the authors. |





| Entry | R 1 | Yield [%] 2 | d.r. (trans:cis) 3 |
|---|---|---|---|
| a | Me | 62 | 62:38 |
| b | cPr | 75 | 64:36 |
| c | cBu | 56 | 62:38 |
| d | tBu | 74 | 54:46 |
| e | nBu | 74 | 67:33 |
| f | iBu | 59 | 69:31 |
| g | cHex | 65 | 67:33 |
| h | Bn | 73 | 62:38 |
| i | (2,6-diMe)Ph | 76 | 62:38 |
| j | (4-MeO)Ph | 46 | 69:31 |
| k 4 | 2-MorphEt | 66 | 72:28 |
| l | EtOOC-CH2- | no reaction | |
| m | Tos-CH2- | no reaction | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Capurro, P.; Moni, L.; Galatini, A.; Mang, C.; Basso, A. Multi-Gram Synthesis of Enantiopure 1,5-Disubstituted Tetrazoles Via Ugi-Azide 3-Component Reaction. Molecules 2018, 23, 2758. https://doi.org/10.3390/molecules23112758
Capurro P, Moni L, Galatini A, Mang C, Basso A. Multi-Gram Synthesis of Enantiopure 1,5-Disubstituted Tetrazoles Via Ugi-Azide 3-Component Reaction. Molecules. 2018; 23(11):2758. https://doi.org/10.3390/molecules23112758
Chicago/Turabian StyleCapurro, Pietro, Lisa Moni, Andrea Galatini, Christian Mang, and Andrea Basso. 2018. "Multi-Gram Synthesis of Enantiopure 1,5-Disubstituted Tetrazoles Via Ugi-Azide 3-Component Reaction" Molecules 23, no. 11: 2758. https://doi.org/10.3390/molecules23112758
APA StyleCapurro, P., Moni, L., Galatini, A., Mang, C., & Basso, A. (2018). Multi-Gram Synthesis of Enantiopure 1,5-Disubstituted Tetrazoles Via Ugi-Azide 3-Component Reaction. Molecules, 23(11), 2758. https://doi.org/10.3390/molecules23112758