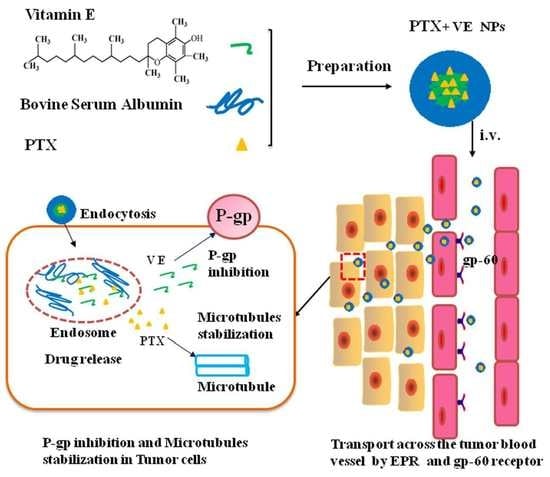
VE-Albumin Core-Shell Nanoparticles for Paclitaxel Delivery to Treat MDR Breast Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Animals
2.2. Preparation of PTX NPs and PTX-VE NPs
2.3. Characterization of PTX-VE NPs
2.3.1. Size Distribution and Morphology
2.3.2. Determination of Entrapment Efficiency (EE) and Loading Capacity (LC)
2.4. Docking Studies
2.5. In Vitro Drug Release
2.6. Cytotoxicity of PTX-VE NPs
2.7. Rhodamine 6G Accumulation in MCF-7/ADR Cells
2.8. Therapeutic Efficacy in Resistant Breast Cancer Xenografts Mice
2.9. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Characterization of PTX-VE NPs
3.2. Docking Studies
3.3. In Vitro Drug Release
3.4. Cytotoxicity of PTX-VE NPs
3.5. Rhodamine 6G Accumulation in MCF-7/ADR Cells
3.6. Anti-Tumor Effect and Safety in the Xenograft Model
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Panchagnula, R. Pharmaceutical aspects of paclitaxel. Int. J. Pharm. 1998, 172, 1–15. [Google Scholar] [CrossRef]
- Sofias, A.M.; Dunne, M.; Storm, G.; Allen, C. The battle of “nano” paclitaxel. Adv. Drug Deliv. Rev. 2017, 122, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Palmeira, A.; Sousa, E.; Vasconcelos, M.H.; Pinto, M.M. Three decades of P-gp inhibitors: Skimming through several generations and scaffolds. Curr. Med. Chem. 2012, 19, 1946–2025. [Google Scholar] [CrossRef] [PubMed]
- Galli, F.; Azzi, A.; Birringer, M.; Cook-Mills, J.M.; Eggersdorfer, M.; Frank, J.; Cruciani, G.; Lorkowski, S.; Özer, N.K. Vitamin E: Emerging aspects and new directions. Free Radic. Biol. Med. 2017, 102, 16–36. [Google Scholar] [CrossRef] [PubMed]
- Meydani, S.N.; Meydani, M.; Rall, L.C.; Morrow, F.; Blumberg, J.B. Assessment of the safety of high-dose, short-term supplementation with vitamin E in healthy older adults. Am. J. Clin. Nutr. 1994, 60, 704–709. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Fu, Q.; Wang, Y.; Racette, K.; Wang, D.; Liu, F. Vitamin E reverses multidrug resistance in vitro and in vivo. Cancer Lett. 2013, 336, 149–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Constantinides, P.P.; Tustian, A.; Kessler, D.R. Tocol emulsions for drug solubilization and parenteral delivery. Adv. Drug Deliv. Rev. 2004, 56, 1243–1255. [Google Scholar] [CrossRef] [PubMed]
- Gou, J.; Feng, S.; Liang, Y.; Fang, G.; Zhang, H.; Yin, T.; Zhang, Y.; He, H.; Wang, Y.; Tang, X. Polyester–solid lipid mixed nanoparticles with improved stability in gastro-intestinal tract facilitated oral delivery of larotaxel. Mol. Pharm. 2017, 14, 3750–3761. [Google Scholar] [CrossRef] [PubMed]
- Gou, Y.; Zhang, Y.; Zhang, Z.; Wang, J.; Zhou, Z.; Liang, H.; Yang, F. Design of an anticancer copper(II) prodrug based on the Lys199 residue of the active targeting human serum albumin nanoparticle carrier. Mol. Pharm. 2017, 14, 1861–1873. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Chen, L.; Zhang, R.; Chen, Z.; Zhu, L. RGD peptide conjugated liposomal drug delivery system for enhance therapeutic efficacy in treating bone metastasis from prostate cancer. J. Control. Release 2014, 196, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Cao, J.; Cui, S.; Qian, Z.; Gu, Y. Enhanced tumor targeting and antitumor efficacy via hydroxycamptothecin-encapsulated folate-modified N-Succinyl-N′-Octyl chitosan micelles. J. Pharm. Sci. 2013, 102, 1318–1332. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Zhang, S.; Ling, Y.; Meng, G.; Yang, Y.; Zhang, W. pH-responsive hybrid quantum dots for targeting hypoxic tumor siRNA delivery. J. Control. Release 2015, 220, 529–544. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-H.; Kankala, R.; Wang, S.-B.; Chen, A.-Z. Leveraging engineering of indocyanine green-encapsulated polymeric nanocomposites for biomedical applications. Nanomaterials 2018, 8, 360. [Google Scholar] [CrossRef] [PubMed]
- Kankala, R.K.; Chen, B.-Q.; Liu, C.-G.; Tang, H.-X.; Wang, S.-B.; Chen, A.-Z. Solution-enhanced dispersion by supercritical fluids: An ecofriendly nanonization approach for processing biomaterials and pharmaceutical compounds. Int. J. Nanomed. 2018, 13, 4227–4245. [Google Scholar] [CrossRef] [PubMed]
- Kankala, R.K.; Zhang, Y.S.; Wang, S.B.; Lee, C.H.; Chen, A.Z. Supercritical Fluid technology: An emphasis on drug delivery and related biomedical applications. Adv. Healthc. Mater. 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.; Tang, B.; Chao, Y.; Xu, H.; Gou, J.; Zhang, Y.; Xu, H.; Tang, X. Cysteine-Functionalized nanostructured lipid carriers for oral delivery of docetaxel: A permeability and pharmacokinetic study. Mol. Pharm. 2015, 12, 2384–2395. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.; Tang, B.; Chao, Y.; Zhang, Y.; Xu, H.; Tang, X. Improved oral bioavailability of docetaxel by nanostructured lipid carriers: In vitro characteristics, in vivo evaluation and intestinal transport studies. RSC Adv. 2015, 5, 96437–96447. [Google Scholar] [CrossRef]
- Fang, G.; Tang, B.; Liu, Z.; Gou, J.; Zhang, Y.; Xu, H.; Tang, X. Novel hydrophobin-coated docetaxel nanoparticles for intravenous delivery: In vitro characteristics and in vivo performance. Eur. J. Pharm. Sci. 2014, 60, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Markman, J.L.; Rekechenetskiy, A.; Holler, E.; Ljubimova, J.Y. Nanomedicine therapeutic approaches to overcome cancer drug resistance. Adv. Drug Deliv. Rev. 2013, 65, 1866–1879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gou, Y.; Zhang, Z.; Li, D.; Zhao, L.; Cai, M.; Sun, Z.; Li, Y.; Zhang, Y.; Khan, H.; Sun, H.; et al. HSA-based multi-target combination therapy: Regulating drugs’ release from HSA and overcoming single drug resistance in a breast cancer model. Drug Deliv. 2018, 25, 321–329. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.U.; Khan, R.U.; Asif, H.; Alamgeer; Khalid, S.H.; Asghar, S.; Saleem, M.; Shah, K.U.; Shah, S.U.; Rizvi, S.A.A.; et al. Co-delivery strategies to overcome multidrug resistance in ovarian cancer. Int. J. Pharm. 2017, 533, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.-S.; Sun, J.-H.; Yu, H.-H.; Yu, S.-Q. Co-delivery nanoparticles of anti-cancer drugs for improving chemotherapy efficacy. Drug Deliv. 2017, 24, 1909–1926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kankala, R.K.; Tsai, P.-Y.; Kuthati, Y.; Wei, P.-R.; Liu, C.-L.; Lee, C.-H. Overcoming multidrug resistance through co-delivery of ROS-generating nano-machinery in cancer therapeutics. J. Mater. Chem. B 2017, 5, 1507–1517. [Google Scholar] [CrossRef]
- Xu, P.-Y.; Kankala, R.K.; Pan, Y.-J.; Yuan, H.; Wang, S.-B.; Chen, A.-Z. Overcoming multidrug resistance through inhalable siRNA nanoparticles-decorated porous microparticles based on supercritical fluid technology. Int. J. Nanomed. 2018, 13, 4685–4698. [Google Scholar] [CrossRef] [PubMed]
- Kankala, R.K.; Liu, C.-G.; Chen, A.-Z.; Wang, S.-B.; Xu, P.-Y.; Mende, L.K.; Liu, C.-L.; Lee, C.-H.; Hu, Y.-F. Overcoming multidrug resistance through the synergistic effects of hierarchical pH-sensitive, ROS-generating nanoreactors. ACS Biomater. Sci. Eng. 2017, 3, 2431–2442. [Google Scholar] [CrossRef]
- Tang, B.; Fang, G.; Gao, Y.; Liu, Y.; Liu, J.; Zou, M.; Cheng, G. Liprosomes loading paclitaxel for brain-targeting delivery by intravenous administration: In vitro characterization and in vivo evaluation. Int. J. Pharm. 2014, 475, 416–427. [Google Scholar] [CrossRef] [PubMed]
- Tang, B.; Fang, G.; Gao, Y.; Liu, Y.; Liu, J.; Zou, M.; Cheng, G. Co-encapsulation of borneol and paclitaxel by liprosomes improved anti-tumor effect in a xenografted glioma model. RSC Adv. 2015, 5, 106613–106620. [Google Scholar] [CrossRef]
- Tang, B.; Fang, G.; Gao, Y.; Liu, Y.; Liu, J.; Zou, M.; Wang, L.; Cheng, G. Lipid-albumin nanoassemblies co-loaded with borneol and paclitaxel for intracellular drug delivery to C6 glioma cells with P-gp inhibition and its tumor targeting. Asian J. Pharm. Sci. 2015, 10, 363–371. [Google Scholar] [CrossRef]
- Guo, Y.; He, W.; Yang, S.; Zhao, D.; Li, Z.; Luan, Y. Co-delivery of docetaxel and verapamil by reduction-sensitive PEG-PLGA-SS-DTX conjugate micelles to reverse the multi-drug resistance of breast cancer. Colloids Surf. B Biointerfaces 2017, 151, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Ruttala, H.B.; Ko, Y.T. Liposomal co-delivery of curcumin and albumin/paclitaxel nanoparticle for enhanced synergistic antitumor efficacy. Colloids Surf. B Biointerfaces 2015, 128, 419–426. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Chen, D.-Y. Application of headspace solid phase microextraction for study of noncovalent interaction of borneol with human serum albumin. Acta Pharm. Sin. 2009, 30, 1573–1576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paal, K.; Müller, J.; Hegedûs, L. High affinity binding of paclitaxel to human serum albumin. Eur. J. Biochem. 2001, 268, 2187–2191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gelderblom, H.; Verweij, J.; Nooter, K.; Sparreboom, A. Cremophor EL: The drawbacks and advantages of vehicle selection for drug formulation. Eur. J. Cancer 2001, 37, 1590–1598. [Google Scholar] [CrossRef]
- Nosrati, H.; Abbasi, R.; Charmi, J.; Rakhshbahar, A.; Aliakbarzadeh, F.; Danafar, H.; Davaran, S. Folic acid conjugated bovine serum albumin: An efficient smart and tumor targeted biomacromolecule for inhibition folate receptor positive cancer cells. Int. J. Biol. Macromol. 2018, 117, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Alexis, F.; Pridgen, E.; Molnar, L.K.; Farokhzad, O.C. Factors affecting the clearance and biodistribution of polymeric nanoparticles. Mol. Pharm. 2008, 5, 505–515. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Yang, Z.; Nie, Y.; Shi, Y.; Fan, D. Multi-drug resistance in cancer chemotherapeutics: Mechanisms and lab approaches. Cancer Lett. 2014, 347, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Krishna, R.; Mayer, L.D. Multidrug resistance (MDR) in cancer: Mechanisms, reversal using modulators of MDR and the role of MDR modulators in influencing the pharmacokinetics of anticancer drugs. Eur. J. Pharm. Sci. 2000, 11, 265–283. [Google Scholar] [CrossRef]
Sample Availability: Samples are not available from the authors. |







| Formulation | Size (nm) | P.I. | Zeta Potential (mV) | EE (%) | LC (%) |
|---|---|---|---|---|---|
| PTX NPs | 101.2 ± 2.8 | 0.167 ± 0.03 | −2.15 ± 0.6 | 91.2 ± 3.0 | 2.5 ± 0.08 |
| PTX-VE NPs | 106.9 ± 3.2 | 0.172 ± 0.02 | −20.64 ± 0.8 | 95.7 ± 2.1 | 12.5 ± 0.15 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, B.; Qian, Y.; Gou, Y.; Cheng, G.; Fang, G. VE-Albumin Core-Shell Nanoparticles for Paclitaxel Delivery to Treat MDR Breast Cancer. Molecules 2018, 23, 2760. https://doi.org/10.3390/molecules23112760
Tang B, Qian Y, Gou Y, Cheng G, Fang G. VE-Albumin Core-Shell Nanoparticles for Paclitaxel Delivery to Treat MDR Breast Cancer. Molecules. 2018; 23(11):2760. https://doi.org/10.3390/molecules23112760
Chicago/Turabian StyleTang, Bo, Yu Qian, Yi Gou, Gang Cheng, and Guihua Fang. 2018. "VE-Albumin Core-Shell Nanoparticles for Paclitaxel Delivery to Treat MDR Breast Cancer" Molecules 23, no. 11: 2760. https://doi.org/10.3390/molecules23112760
APA StyleTang, B., Qian, Y., Gou, Y., Cheng, G., & Fang, G. (2018). VE-Albumin Core-Shell Nanoparticles for Paclitaxel Delivery to Treat MDR Breast Cancer. Molecules, 23(11), 2760. https://doi.org/10.3390/molecules23112760