Curvulin and Phaeosphaeride A from Paraphoma sp. VIZR 1.46 Isolated from Cirsium arvense as Potential Herbicides
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fungal Identification
2.2. Purification and Identification of Phytotoxins
2.3. Biological Activity
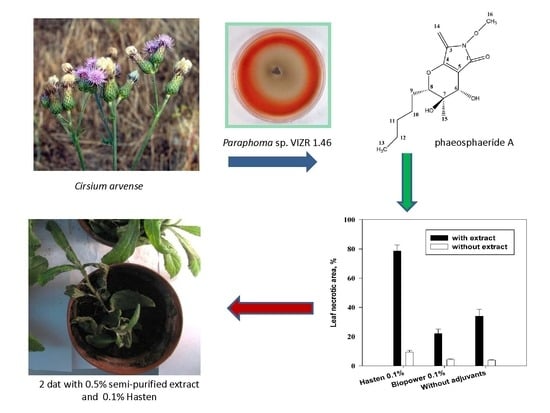
2.4. Effects of Leaf Wounding, Solvent and Adjuvants on Phytotoxic Activity of Phaeosphaeride A
2.5. Effect of Adjuvants on Herbicidal Activity of Phaeosphaeride A
3. Material and Methods
3.1. General Experimental Procedures
3.2. Fungal Strain
3.3. Production and Purification of Phytotoxic Metabolites
3.3.1. Liquid Culture
3.3.2. Solid Culture
3.4. Compound Characterization
3.5. Biological Assays
3.6. Effects of Leaf Wounding, Solvents and Adjuvants on Phytotoxic Activity of Phaeosphaeride A
3.7. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Patents
References
- Nicolopoulou-Stamati, P.; Maipas, S.; Kotampasi, C.; Stamatis, P.; Hens, L. Chemical pesticides and human health: The urgent need for a new concept in agriculture. Front. Public Health 2016, 4, 148. [Google Scholar] [CrossRef] [PubMed]
- Westwood, J.H.; Charudattan, R.; Duke, S.O.; Fennimore, S.A.; Marrone, P.; Slaughter, D.C.; Swanton, C.; Zollinger, R. Weed management in 2050: Perspectives on the future of weed science. Weed Sci. 2018, 66, 275–285. [Google Scholar] [CrossRef]
- Sparks, T.C.; Hahn, D.R.; Garizi, N.V. Natural products, their derivatives, mimics and synthetic equivalents: Role in agrochemical discovery. Pest Manag. Sci. 2017, 73, 700–715. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Qiang, S. Recent advances in tenuazonic acid as a potential herbicide. Pesticide Biochem. Physiol. 2017, 143, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Duke, S.O.; Dayan, F.E. Discovery of new herbicide modes of action with natural phytotoxins. In Discovery and Synthesis of Crop Protection Products; Maienfisch, P., Stevenson, T.M., Eds.; American Chemical Society: Washington, DC, USA, 2015; pp. 79–92. ISBN 9780841231023. [Google Scholar]
- Cimmino, A.; Masi, M.; Evidente, M.; Superchi, S.; Evidente, A. Fungal phytotoxins with potential herbicidal activity: Chemical and biological characterization. Nat. Prod. Rep. 2015, 32, 1629–1653. [Google Scholar] [CrossRef] [PubMed]
- Pusztahelyi, T.; Holb, I.J.; Pócsi, I. Secondary metabolites in fungus-plant interactions. Front. Plant Sci. 2015, 6, 1–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berestetskiy, A.O. A review of fungal phytotoxins: From basic studies to practical use. Appl. Biochem. Microbiol. 2008, 44, 453–465. [Google Scholar] [CrossRef]
- Rivero-Cruz, F.J.; García-Aguirre, G.; Cerda-García-Rojas, C.M.; Mata, R. Conformational behavior and absolute stereostructure of two phytotoxic nonenolides from the fungus Phoma herbarum. Tetrahedron 2000, 56, 5337–5344. [Google Scholar] [CrossRef]
- Graupner, P.R.; Carr, A.; Clancy, E.; Gilbert, J.; Bailey, K.L.; Derby, J.; Gerwick, B.C. The Macrocidins: Novel cyclic tetramic acids with herbicidal activity produced by Phoma macrostoma. J. Nat. Prod. 2003, 66, 1558–1561. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, A.; Andolfi, A.; Berestetskiy, A.; Evidente, A. Production of phytotoxins by Phoma exigua var. exigua, a potential mycoherbicide against perennial thistles. J. Agric. Food Chem. 2008, 56, 6304–6309. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, A.; Andolfi, A.; Zonno, M.C.; Avolio, F.; Berestetskiy, A.; Vurro, M.; Evidente, A. Chenopodolans A-C: Phytotoxic furopyrans produced by Phoma chenopodiicola, a fungal pathogen of Chenopodium album. Phytochemistry 2013, 96, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Cimmino, A.; Andolfi, A.; Zonno, M.C.; Avolio, F.; Santini, A.; Tuzi, A.; Berestetskyi, A.; Vurro, M.; Evidente, A. Chenopodolin: A phytotoxic unrearranged ent-pimaradiene diterpene produced by Phoma chenopodicola, a fungal pathogen for Chenopodium album biocontrol. J. Nat. Prod. 2013, 76, 1291–1297. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.C. Combinatorial chemistry in the development of new crop protection products. Pestic. Outlook 2003, 14, 21–25. [Google Scholar] [CrossRef]
- Berestetskii, A.O.; Yuzikhin, O.S.; Katkova, A.S.; Dobrodumov, A.V.; Sivogrivov, D.E.; Kolombet, L.V. Isolation, identification, and characteristics of the phytotoxin produced by the fungus Alternaria cirsinoxia. Appl. Biochem. Microbiol. 2010, 46, 75–79. [Google Scholar] [CrossRef]
- Fumagalli, P.; Andolfi, A.; Avolio, F.; Boari, A.; Cimmino, A.; Finizio, A. Ecotoxicological characterisation of a mycoherbicide mixture isolated from the fungus Ascochyta caulina. Pest Manag. Sci. 2013, 9, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Radivojević, L.; Gašić, S.; Umiljendić, J.G.; Šantrić, L.; Brkić, D. Impact of Different Adjuvants and Modes of Application on Efficacy of Rimsulfuron in Maize. Pestic. Phytomed. 2011, 26, 255–263. [Google Scholar] [CrossRef]
- Yilmaz, G.; Dane, F. Phytotoxicity Induced by Herbicide and Surfactant on stomata and epicuticular wax of Wheat. Rom. Biotechnol. Lett. 2012, 17, 7757–7765. [Google Scholar]
- Gitsopoulos, T.K.; Damalas, C.A.; Georgoulas, I. Improving diquat efficacy on grasses by adding adjuvants to the spray solution before use. Planta Daninha 2014, 32, 355–360. [Google Scholar] [CrossRef] [Green Version]
- Gurtovenko, A.; Anwar, J. Modulating the structure and properties of cell membranes: The molecular mechanism of action of dimethyl sulfoxide. J. Phys. Chem. B 2007, 111, 10453–10460. [Google Scholar] [CrossRef] [PubMed]
- Berestetskyi, A.O.; Kurlenya, A.S. Antimicrobial properties of phytopathogenic mycromycetes. Mycol. Phytopathol. 2014, 48, 123–134. [Google Scholar]
- Phookamsak, R.; Liu, J.; McKenzie, E.H.C.; Manamgoda, D.S.; Ariyawansa, H.; Thambugala, K.M.; Dai, D.; Camporesi, E.; Chukeatirote, E.; Wijayawardene, N.N.; et al. Revision of Phaeosphaeriaceae. Fungal Divers. 2014, 68, 159–238. [Google Scholar] [CrossRef]
- Moslemi, A.; Ades, P.K.; Groom, T.; Crous, P.W.; Nicolas, M.E.; Taylor, P.W.J. Paraphoma Crown Rot of Pyrethrum (Tanacetum cinerariifolium). Plant Dis. 2016, 100, 2363–2369. [Google Scholar] [CrossRef]
- Boerema, G.H.; Gruyter, J.; Noordeloos, M.E.; Hamers, M.A. Phoma Identification Manual: Differentiation of Specific and Infraspecific Taxa in Culture; CABI Publishing: Wallingford/Cambridge, UK, 2004; p. 470. [Google Scholar]
- Kluth, S.; Kruess, A.; Tscharntke, T. Effects of two pathogens on the performance of Cirsium arvense in a successional fallow. Weed Res. 2005, 45, 261–269. [Google Scholar] [CrossRef]
- Evidente, A.; Cimmino, A.; Andolfi, A.; Vurro, M.; Zonno, M.C.; Cantrell, C.L.; Motta, A. Phyllostictines A-D, oxazatricycloalkenones produced by Phyllosticta cirsii, a potential mycoherbicide for Cirsium arvense biocontrol. Tetrahedron 2008, 64, 1612–1619. [Google Scholar] [CrossRef]
- Evidente, A.; Cimmino, A.; Berestetskiy, A.; Andolfi, A.; Motta, A. Stagonolides G-I and modiolide A, nonenolides produced by Stagonospora cirsii, a potential mycoherbicide for Cirsium arvense. J. Nat. Prod. 2008, 71, 1897–1901. [Google Scholar] [CrossRef] [PubMed]
- Trenti, F.; Cox, R.J. Structural revision and biosynthesis of the fungal phytotoxins phyllostictines A and B. J. Nat. Prod. 2017, 80, 1235–1240. [Google Scholar] [CrossRef] [PubMed]
- Kenfield, D.; Hallok, Y.; Clard, J.; Strobel, G. Curvulin and o–methylcurvulinic acid: Phytotoxic metabolites of Dreshlera indica which cause necroses on purslane and spiny amaranth. Plant Sci. 1989, 60, 123–127. [Google Scholar] [CrossRef]
- Kamal, A.; Khan, M.A.; Qureshi, A. Studies in the biochemistry of microorganisms–II Constitution of curvulin, curvulinic acid and curvulol, metabolic products of Curvularia siddiqui. Tetrahedron 1963, 19, 111–115. [Google Scholar] [CrossRef]
- Kamal, A.; Ahmad, N.; Ali Khan, M.; Qureshi, H. Studies in the biochemistry of microorganisms—I. Curvulin and curvulinic acid, metabolic products of Curvularia siddiqui. Tetrahedron 1962, 18, 433–436. [Google Scholar] [CrossRef]
- Varma, G.B.; Fatope, M.O.; Marwah, R.G.; Deadman, M.E.; Al–Rawahi, F.K. Production of phenylacetic acid derivatives and 4–epiradicinol in culture by Curvularia lunata. Phytochemistry 2006, 67, 1925–1930. [Google Scholar] [CrossRef] [PubMed]
- de Almeida, T.T.; Ribeiro, M.A.; Polonio, J.C.; Garcia, F.P.; Nakamura, C.V.; Meurer, E.C.; Sarragiotto, M.H.; Baldoqui, D.C.; Azevedo, J.L.; Pamphile, J.A. Curvulin and spirostaphylotrichins R and U from extracts produced by two endophytic Bipolaris sp. associated to aquatic macrophytes with antileishmanial activity. Nat. Prod. Res. 2017, 26, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Maloney, K.N.; Hao, W.; Xu, J.; Gibbons, J.; Hucul, J.; Roll, D.; Brady, S.F.; Schroeder, F.C.; Clardy, J. Phaeosphaeride A, an inhibitor of STAT3-dependent signaling isolated from an endophytic fungus. Org. Lett. 2006, 4067–4070. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.; Braun, S. 200 and More Basic NMR Experiments: A Practical Course, 1st ed; Wiley-VCH: Weinheim, Germany, 2004; p. 838. ISBN 3-527-31067-3. [Google Scholar]
- Kobayashi, K.; Okamoto, I.; Morita, N.; Kiyotani, T.; Tamura, O. Synthesis of the proposed structure of phaeosphaeride A. Org. Biomol. Chem. 2011, 9, 5825–5832. [Google Scholar] [CrossRef] [PubMed]
- Chatzimpaloglou, A.; Yavropoulou, M.P.; Rooij, K.E.; Biedermann, R.; Mueller, U.; Kaskel, S.; Sarli, V. Total synthesis and biological activity of the proposed structure of phaeosphaeride A. J. Org. Chem. 2012, 77, 9659–9667. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Kobayashi, Y.; Nakamura, M.; Tamura, O.; Kogen, H. Establishment of relative and absolute configurations of phaeosphaeride A: Total synthesis of ent-phaeosphaeride A. J. Org. Chem. 2015, 80, 1243–1248. [Google Scholar] [CrossRef] [PubMed]
- Abzianidze, V.V.; Efimova, K.P.; Poluektova, E.V.; Trishin, Y.G.; Kuznetsov, V.A. Synthesis of natural phaeosphaeride A and semi-natural phaeosphaeride B derivatives. Mendeleev Commun. 2017, 27, 490–492. [Google Scholar] [CrossRef]
- Abzianidze, V.V.; Poluektova, E.V.; Bolshakova, K.P.; Panikorovskii, T.L.; Bogachenkov, A.S.; Berestetskiy, A.O. Crystal structure of natural phaeosphaeride A. Acta Cryst. 2015, E71, o625. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Tanaka, K.; Kogen, H. Total Synthesis and Biological Evaluation of Phaeosphaerides. Catalysts 2018, 8, 206. [Google Scholar] [CrossRef]
- Masi, M.; Meyer, S.; Clement, S.; Andolfi, A.; Cimmino, A.; Evidente, A. Spirostaphylotrichin W, a spirocyclic γ-lactam isolated from liquid culture of Pyrenophora semeniperda, a potential mycoherbicide for cheatgrass (Bromus tectorum) biocontrol. Tetrahedron 2014, 70, 1497–1501. [Google Scholar] [CrossRef]
- Abraham, W.R.; Meyer, M. Curvupallides, a new class of alkaloids from the fungus Curvularia pallescens. Tetrahedron 1995, 51, 4947–4952. [Google Scholar] [CrossRef]
- Li, C.S.; Ding, Y.; Yang, B.J.; Miklossy, G.; Yin, H.Q.; Walker, L.A.; Turkson, J.; Cao, S. A new metabolite with a unique 4-pyranone−γ-lactam−1,4-thiazine moiety from a hawaiian-plant associated fungus. Org. Lett. 2015, 17, 3556–3559. [Google Scholar] [CrossRef] [PubMed]
- Yuzikhin, O.; Mitina, G.; Berestetskiy, A. Herbicidal potential of stagonolide, a new phytotoxic nonenolide from Stagonospora cirsii. J. Agric. Food Chem. 2007, 55, 7707–7711. [Google Scholar] [CrossRef] [PubMed]
- Field, R.J.; Dobson, N.N.; Tisdall, L.J. Species-specific sensitivity to organosilicone surfactant-enhancement of glyphosate uptake. In Adjuvants for Agrichemicals; Foy, C.L., Ed.; CRC Press, Inc.: Boca Raton, FL, USA, 1992; pp. 423–431. ISBN 9781351077958. [Google Scholar]
- Webber, C.L., III; White, P.M., Jr.; Shrefler, J.W.; Spaunhorst, D.J. Impact of acetic acid concentration, application volume, and adjuvants on weed control efficacy. J. Agric. Sci. 2018, 10, 1–6. [Google Scholar] [CrossRef]
- Renner, K.A. Canada thistle (Cirsium arvense) control in sugarbeet with clopyralid. Weed Technol. 1991, 5, 392–395. [Google Scholar] [CrossRef]
- Zollinger, R.K. Influence of adjuvants on weed control from tribenuron. J. ASTM Int. 2005, 2, 1–7. [Google Scholar] [CrossRef]
- Lockett, J.; Morgan, C. Herbicide Composition. European Patent N 1981339B1, 29 February 2012. Available online: https://patents.google.com/patent/EP1981339B1/zh-cn (accessed on 22 October 2018).
- Fanigliulo, A.; Filì, V.; Crescenzi, A. Evaluation of efficacy and effect of application timing of a new herbicide, a.i. propoxy- carbazone + iodosulfuron + mefenpyr on Triticum durum. Commun. Agric. Appl. Biol. Sci. 2012, 77, 483–488. [Google Scholar] [PubMed]
- Papapanagiotou, A.P.; Kaloumenos, N.S.; Eleftherohorinos, I.G. Sterile oat (Avena sterilis L.) cross-resistance profile to ACCase-inhibiting herbicides in Greece. Crop Prot. 2012, 35, 118–126. [Google Scholar] [CrossRef]
- Ferguson, J.C.; Chechetto, R.G.; Adkins, S.W.; Hewitt, A.J.; Chauhan, B.S.; Kruger, G.R.; O’Donnell, C.C. Effect of spray droplet size on herbicide efficacy on four winter annual grasses. Crop Prot. 2018, 112, 118–124. [Google Scholar] [CrossRef]
- Sambrook, E.A.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1989; p. 479. [Google Scholar]
- Rehner, S.A.; Samuels, G.J. Taxonomy and phylogeny of Gliocladium analysed from nuclear large subunit ribosomal DNA sequences. Mycol. Res. 1994, 98, 625–634. [Google Scholar] [CrossRef]
- Vilgalys, R.; Hester, M. Rapid genetic identification and mapping of enzymatically amplified ribosomal DNA from several Cryptococcus species. J. Bacteriol. 1990, 172, 4238–4246. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: New York, NY, USA, 1990; pp. 315–322. [Google Scholar]
- Arullappan, S.; Zakaria, Z.; Basri, D.F. Preliminary screening of antibacterial activity using crude extracts of Hibiscus rosa sinensis. Trop Life Sci. Res. 2009, 20, 109–118. [Google Scholar] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poluektova, E.; Tokarev, Y.; Sokornova, S.; Chisty, L.; Evidente, A.; Berestetskiy, A. Curvulin and Phaeosphaeride A from Paraphoma sp. VIZR 1.46 Isolated from Cirsium arvense as Potential Herbicides. Molecules 2018, 23, 2795. https://doi.org/10.3390/molecules23112795
Poluektova E, Tokarev Y, Sokornova S, Chisty L, Evidente A, Berestetskiy A. Curvulin and Phaeosphaeride A from Paraphoma sp. VIZR 1.46 Isolated from Cirsium arvense as Potential Herbicides. Molecules. 2018; 23(11):2795. https://doi.org/10.3390/molecules23112795
Chicago/Turabian StylePoluektova, Ekaterina, Yuriy Tokarev, Sofia Sokornova, Leonid Chisty, Antonio Evidente, and Alexander Berestetskiy. 2018. "Curvulin and Phaeosphaeride A from Paraphoma sp. VIZR 1.46 Isolated from Cirsium arvense as Potential Herbicides" Molecules 23, no. 11: 2795. https://doi.org/10.3390/molecules23112795
APA StylePoluektova, E., Tokarev, Y., Sokornova, S., Chisty, L., Evidente, A., & Berestetskiy, A. (2018). Curvulin and Phaeosphaeride A from Paraphoma sp. VIZR 1.46 Isolated from Cirsium arvense as Potential Herbicides. Molecules, 23(11), 2795. https://doi.org/10.3390/molecules23112795