Galactosylated Chitosan-Functionalized Mesoporous Silica Nanoparticle Loading by Calcium Leucovorin for Colon Cancer Cell-Targeted Drug Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of MSN–COOH–GC and MSN–COOH/GC
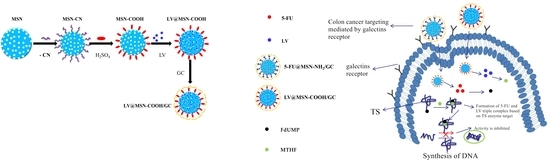
2.2.1. Synthesis of Carboxyl Functionalized Mesoporous Silica Nanoparticles (MSN–COOH)
2.2.2. Synthesis of Galactosylated Chitosan (GC)
2.2.3. Synthesis of MSN–COOH–GC
2.2.4. Synthesis of MSN–COOH/GC
2.3. Preparation of MSN–COOH-Loaded LV
2.4. Preparation of MSN–COOH/GC-Loaded LV
2.5. Preparation of MSN–COOH–GC-Loaded LV
2.6. MSN–COOH and MSN–COOH/GC Zeta Potential (ς)
2.7. Morphology Observations
2.8. Other Characterizations
2.9. In Vitro Drug Release
2.10. Cell Culture
2.11. Determination of Biocompatibility of LV, MSN–COOH, and MSN–COOH/GC
2.12. Cytotoxicity Assay of Combined Action of 5-FU, 5-FU@MSN–NH2/GC, and LV@MSN–COOH/GC
2.13. Uptake Experiment
2.13.1. Fluorescence Microscopy Cell Uptake
2.13.2. Flow Cytometry Cell Uptake
2.14. Human Thymidylate Synthase (TS) Enzyme-Linked Immunosorbent Assay (ELISA)
2.15. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Nanocarriers
3.2. In Vitro Antitumor Activity of LV@MSN–NH2/GC
3.2.1. LV, MSN–COOH, and MSN–COOH/GC Biocompatibility Determination
3.2.2. Cytotoxicity Assay of the Combined Action of 5-FU, 5-FU@MSN–NH2/GC, and LV@MSN–COOH/GC
3.2.3. Uptake Assay
Fluorescence Microscopy Cell Uptake
Flow Cytometry Cell Uptake
3.2.4. Human Thymidylate Synthase (TS) Enzyme-Linked Immunosorbent Assay (ELISA)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, W.Q.; Zheng, R.S.; Zeng, H.M. Report of Cancer Incidence and Mortality in China. Chin. J. Oncol. 2014, 23, 1–10. [Google Scholar]
- Safwat, M.A.; Soliman, G.M.; Sayed, D.; Attia, M.A. Gold nanoparticles enhance 5-fluorouracil anticancer efficacy against colorectal cancer cells. Int. J. Pharm. 2016, 513, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Shivakumar, H.G.; Fathima, S.J.; Radha, V.; Khanum, F. pH and thermosensitive 5-fluorouracil loaded poly(NIPAM-co-AAc) nanogels for cancer therapy. RSC Adv. 2016, 6, 105495–105507. [Google Scholar] [CrossRef]
- Paul Spears, C.; Malcolm, B.G.; Mitchell, M.S.; Spicer, D.; Berne, M.; Bernstein, L.; Danenberg, P.V. Thymidylate synthetase inhibition in malignant tumors and normal liver of patients given intravenous 5-fluorouracil. Cancer Res. 1984, 44, 4144–4150. [Google Scholar]
- Nakamura, A.; Nakajima, G.; Okuyama, R.; Kuramochi, H.; Kondoh, Y.; Kanemura, T.; Takechi, T.; Yamamoto, M.; Hayashi, K. Enhancement of 5-fluorouracil-induced cytotoxicity by leucovorin in 5-fluorouracil-resistant gastric cancer cells with upregulated expression of thymidylate synthase. Gastric Cancer 2014, 17, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Santi, D.V.; Mchenry, C.S.; Sommer, H. Mechanism of interaction of thymidylate synthetase with 5-fluorodeoxyuridylate. Biochemistry 1974, 13, 471–481. [Google Scholar] [CrossRef] [PubMed]
- Tsujimoto, H.; Tsukioka, S.; Ono, S.; Sakamoto, E.; Sakamoto, K.; Tsuta, K.; Nakagawa, F.; Saito, H.; Uchida, J.; Kiniwa, M.; et al. Effect of leucovorin on the antitumor efficacy of the 5-FU prodrug, tegafur-uracil, in human colorectal cancer xenografts with various expression levels of thymidylate synthase. Oncol. Lett. 2010, 1, 973–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benson, A.B., III; Venook, A.P.; Cederquist, L.; Chan, E.; Chen, Y.; Cooper, H.S.; Deming, D.; Engstrom, P.F.; Engzinger, P.C.; Fichera, A.; et al. Colon Cancer, Version 1.2017 Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 2017, 15, 370–398. [Google Scholar] [CrossRef]
- Gustavsson, B.; Carlsson, G.; Machover, D.; Petrelli, N.; Roth, A.; Schmoll, H.-J.; Tveit, K.-M.; Gibson, F. A Review of the Evolution of Systemic Chemotherapy in the Management of Colorectal Cancer. Clin. Colorectal Cancer 2015, 14, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Wolpin, B.M.; Mayer, R.J. Systemic treatment of colorectal cancer. Gastroenterology 2008, 134, 1296–1310. [Google Scholar] [CrossRef] [PubMed]
- Moynihan, T.; Hansen, R.; Anderson, T.; Quebbeman, E.; Beatty, P.; Ausman, R.; Ritch, P.; Chitambar, C.; Vukelich, M. Continuous 5-fluorouracil infusion in advanced gastric carcinoma. Am. J. Clin. Oncol. 1988, 11, 461–464. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Hu, H.; He, L.; Chen, T. Differential Effects of Polymer-Surface Decoration on Drug Delivery, Cellular Retention, and Action Mechanisms of Functionalized Mesoporous Silica Nanoparticles. Chem. Asian J. 2015, 10, 2744–2754. [Google Scholar] [CrossRef] [PubMed]
- Lamprecht, A.; Yamamoto, H.; Takeuchi, H.; Kawashima, Y. Observations in simultaneous microencapsulation of 5-fluorouracil and leucovorin for combined pH-dependent release. Eur. J. Pharm. Biopharm. 2005, 59, 367–371. [Google Scholar] [CrossRef] [PubMed]
- Li, P.W.; Wang, Y.C.; Peng, Z.; She, F.H.; Kong, L.X. Development of chitosan nanoparticles as drug delivery systems for 5-fluorouracil and leucovorin blends. Carbohydr. Polym. 2011, 85, 698–704. [Google Scholar] [CrossRef]
- Li, P.W.; Yang, Z.M.; Wang, Y.C.; Peng, Z.; Li, S.D.; Kong, L.X.; Wang, Q.H. Microencapsulation of coupled folate and chitosan nanoparticles for targeted delivery of combination drugs to colon. J. Microencapsul. 2015, 32, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Anirudhan, T.S.; Vasantha, C.S.; Sasidharan, A.V. Layer-by-layer assembly of hyaluronic acid/carboxymethylchitosan polyelectrolytes on the surface of aminated mesoporous silica for the oral delivery of 5-fluorouracil. Eur. Polym. J. 2017, 93, 572–589. [Google Scholar] [CrossRef]
- Fang, S.; Lin, J.; Li, C.; Huang, P.; Hou, W.; Zhang, C.; Liu, J.; Huang, S.; Luo, Y.; Fan, W.; et al. Dual-Stimuli Responsive Nanotheranostics for Multimodal Imaging Guided Trimodal Synergistic Therapy. Small 2017, 13. [Google Scholar] [CrossRef] [PubMed]
- Kumar, B.; Kulanthaivel, S.; Mondal, A.; Mishra, S.; Banerjee, B.; Bhaumik, A.; Banerjee, I.; Giri, S. Mesoporous silica nanoparticle based enzyme responsive system for colon specific drug delivery through guar gum capping. Colloids Surf. B Biointerfaces 2017, 150, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Liu, Q.; Wang, L.; Jiang, W.; Zhang, W.; Song, X. Multifunctional triple-porous Fe3O4@SiO2 superparamagnetic microspheres for potential hyperthermia and controlled drug release. RSC Adv. 2017, 7, 32049–32057. [Google Scholar] [CrossRef]
- Vilaça, N.; Machado, A.F.; Morais-Santos, F.; Amorim, R.; Patrícia Neto, A.; Logodin, E.; Pereira, M.F.R.; Sardo, M.; Rocha, J.; Parpot, P.; et al. Comparison of different silica microporous structures as drug delivery systems for in vitro models of solid tumors. RSC Adv. 2017, 7, 13104–13111. [Google Scholar] [CrossRef] [Green Version]
- Egodawatte, S.; Dominguez, S.; Larsen, S.C. Solvent effects in the development of a drug delivery system for 5-fluorouracil using magnetic mesoporous silica nanoparticles. Microporous Mesoporous Mater. 2017, 237, 108–116. [Google Scholar] [CrossRef]
- Giret, S.; Theron, C.; Gallud, A.; Maynadier, M.; Gary-Bobo, M.; Garcia, M.; Wong Chi Man, M.; Carcel, C. A designed 5-fluorouracil-based bridged silsesquioxane as an autonomous acid-triggered drug-delivery system. Chemistry 2013, 19, 12806–12814. [Google Scholar] [CrossRef] [PubMed]
- Mathew, A.; Parambadath, S.; Park, S.S.; Ha, C.-S. Hydrophobically modified spherical MCM-41 as nanovalve system for controlled drug delivery. Microporous Mesoporous Mater. 2014, 200, 124–131. [Google Scholar] [CrossRef]
- Shen, B.; Ma, Y.; Yu, S.; Ji, C. Smart Multifunctional Magnetic Nanoparticle-Based Drug Delivery System for Cancer Thermo-Chemotherapy and Intracellular Imaging. ACS Appl. Mater. Interfaces 2016, 8, 24502–24508. [Google Scholar] [CrossRef] [PubMed]
- Tomoiaga, A.M.; Cioroiu, B.I.; Nica, V.; Vasile, A. Investigations on nanoconfinement of low-molecular antineoplastic agents into biocompatible magnetic matrices for drug targeting. Colloids Surf. B Biointerfaces 2013, 111, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, M.S.; Bae, J.-H.; Kim, M.-J.; Kim, S.-H.; Ha, C.-S. Design of a Novel Mesoporous Organosilica Hybrid Microcarrier: A pH Stimuli-Responsive Dual-Drug-Delivery Vehicle for Intracellular Delivery of Anticancer Agents. Part. Part. Syst. Charact. 2013, 30, 1044–1055. [Google Scholar] [CrossRef]
- Murugan, B.; Narashimhan Ramana, L.; Gandhi, S.; Sethuraman, S.; Krishnan, U.M. Engineered chemoswitchable mesoporous silica for tumor-specific cytotoxicity. J. Mater. Chem. B 2013, 1, 3494. [Google Scholar] [CrossRef]
- Chen, L.; She, X.; Wang, T.; He, L.; Shigdar, S.; Duan, W.; Kong, L. Overcoming acquired drug resistance in colorectal cancer cells by targeted delivery of 5-FU with EGF grafted hollow mesoporous silica nanoparticles. Nanoscale 2015, 7, 14080–14092. [Google Scholar] [CrossRef] [PubMed]
- She, X.; Chen, L.; Velleman, L.; Li, C.; Zhu, H.; He, C.; Wang, T.; Shigdar, S.; Duan, W.; Kong, L. Fabrication of high specificity hollow mesoporous silica nanoparticles assisted by Eudragit for targeted drug delivery. J. Colloid Interface Sci. 2015, 445, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.J.; Hsu, W.Y. Pegylation effect of chitosan based polyplex on DNA transfection. Carbohydr. Polym. 2015, 120, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yu, Y.Y.; Li, D.; Peng, R.; Li, Y.; Jiang, Q.; Dai, P.; Gao, R. Synthesis and properties of a novel methoxy poly(ethylene glycol)-modified galactosylated chitosan derivative. J. Mater. Sci. Mater. Med. 2009, 20, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Zhang, W.; Peng, R.; Huang, J.; Nie, T.; Li, Y.; Jiang, Q.; Gao, R. Synthesis and cell activity of novel galactosylated chitosan as a gene carrier. Colloids Surf. B Biointerfaces 2009, 70, 181–186. [Google Scholar] [CrossRef] [PubMed]
- D’Souza, A.A.; Devarajan, P.V. Asialoglycoprotein receptor mediated hepatocyte targeting-strategies and applications. J. Control. Release 2015, 203, 126–139. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Fu, W.; Liao, M.; Han, B.; Chang, J.; Yang, Y. Construction and characterization of Gal-chitosan graft methoxy poly (ethylene glycol) (Gal-CS-mPEG) nanoparticles as efficient gene carrier. J. Ocean. Univ. China 2017, 16, 873–881. [Google Scholar] [CrossRef]
- Zhou, N.; Zan, X.; Wang, Z.; Wu, H.; Yin, D.; Liao, C.; Wan, Y. Galactosylated chitosan-polycaprolactone nanoparticles for hepatocyte-targeted delivery of curcumin. Carbohydr. Polym. 2013, 94, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Tang, C.; Yin, C. Galactosylated trimethyl chitosan-cysteine nanoparticles loaded with Map4k4 siRNA for targeting activated macrophages. Biomaterials 2013, 34, 3667–3677. [Google Scholar] [CrossRef] [PubMed]
- Zuo, L.; Huang, Z.; Dong, L.; Xu, L.; Zhu, Y.; Zeng, K.; Zhang, C.; Chen, J.; Zhang, J. Targeting delivery of anti-TNFalpha oligonucleotide into activated colonic macrophages protects against experimental colitis. Gut 2010, 59, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Barrow, H.; Rhodes, J.M.; Yu, L.G. The role of galectins in colorectal cancer progression. Int. J. Cancer 2011, 129, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gopalan, V.; Saremi, N.; Sullivan, E.; Kabir, S.; Lu, C.T.; Salajegheh, A.; Leung, M.; Smith, R.A.; Lam, A.K. The expression profiles of the galectin gene family in colorectal adenocarcinomas. Hum. Pathol. 2016, 53, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Barondes, S.H.; Cooper, D.N.; Gitt, M.A.; Leffle, H. Structure and function of a large family of animal lectins. J. Biol. Chem. 1991, 269, 20807–20810. [Google Scholar]
- Kornarakis, I.; Sopasis, G.; Milios, C.J.; Armatas, G.S. Incorporation of a high-spin heptanuclear [CuII6Gd] cluster into carboxyl-functionalized mesoporous silica. RSC Adv. 2012, 2, 9809–9815. [Google Scholar] [CrossRef]
- Moorthy, M.S.; Park, J.-H.; Bae, J.-H.; Kim, S.-H.; Ha, C.-S. Mesoporous organosilica hybrids with a tunable amphoteric framework for controlled drug delivery. J. Mater. Chem. B 2014, 2, 6487–6499. [Google Scholar] [CrossRef]
- Shen, Y.; Zhang, Y.; Zhang, X.; Zhou, X.; Teng, X.; Yan, M.; Bi, H. Horseradish peroxidase-immobilized magnetic mesoporous silica nanoparticles as a potential candidate to eliminate intracellular reactive oxygen species. Nanoscale 2015, 7, 2941–2950. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Shi, H.; Li, Z.; Shen, H.; Ma, K.; Li, B.; Shen, S.; Jin, Y. A multifunctional mesoporous silica nanocomposite for targeted delivery, controlled release of doxorubicin and bioimaging. Colloids Surf. B Biointerfaces 2013, 110, 138–147. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Park, S.S.; Yun, Y.H.; Ha, C.-S. Mesoporous silica nanoparticles functionalized with a redox-responsive biopolymer. J. Porous Mater. 2017, 24, 1215–1225. [Google Scholar] [CrossRef]
- Tian, B.; Liu, S.; Wu, S.; Lu, W.; Wang, D.; Jin, L.; Hu, B.; Li, K.; Wang, Z.; Quan, Z. pH-responsive poly (acrylic acid)-gated mesoporous silica and its application in oral colon targeted drug delivery for doxorubicin. Colloids Surf. B Biointerfaces 2017, 154, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Li, T.; Zheng, G.; Jiang, K.; Fan, L.; Shao, J. Simultaneous inhibition of growth and metastasis of hepatocellular carcinoma by co-delivery of ursolic acid and sorafenib using lactobionic acid modified and pH-sensitive chitosan-conjugated mesoporous silica nanocomplex. Biomaterials 2017, 143, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Li, Y.; Niu, D.; Li, L.; Zhao, W.; Chen, H.; Li, L.; Gao, J.; Ruan, M.; Shi, J. A facile route to hollow nanospheres of mesoporous silica with tunable size. Chem. Commun. Camb. 2008, 2629–2631. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Shi, J.; Chen, F.; Zhu, M.; Zhang, L. An anticancer drug delivery system based on surfactant-templated mesoporous silica nanoparticles. Biomaterials 2010, 31, 3335–3346. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, C.Q. pH and redox-operated nanovalve for size-selective cargo delivery on hollow mesoporous silica spheres. J. Colloid Interface Sci. 2016, 480, 39–48. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds not available are from the authors. |
















© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Wang, F.; Zhu, Y.; Li, X.; Liu, X.; Pang, J.; Pan, W. Galactosylated Chitosan-Functionalized Mesoporous Silica Nanoparticle Loading by Calcium Leucovorin for Colon Cancer Cell-Targeted Drug Delivery. Molecules 2018, 23, 3082. https://doi.org/10.3390/molecules23123082
Liu W, Wang F, Zhu Y, Li X, Liu X, Pang J, Pan W. Galactosylated Chitosan-Functionalized Mesoporous Silica Nanoparticle Loading by Calcium Leucovorin for Colon Cancer Cell-Targeted Drug Delivery. Molecules. 2018; 23(12):3082. https://doi.org/10.3390/molecules23123082
Chicago/Turabian StyleLiu, Wei, Fan Wang, Yongchao Zhu, Xue Li, Xiaojing Liu, Jingjing Pang, and Weisan Pan. 2018. "Galactosylated Chitosan-Functionalized Mesoporous Silica Nanoparticle Loading by Calcium Leucovorin for Colon Cancer Cell-Targeted Drug Delivery" Molecules 23, no. 12: 3082. https://doi.org/10.3390/molecules23123082
APA StyleLiu, W., Wang, F., Zhu, Y., Li, X., Liu, X., Pang, J., & Pan, W. (2018). Galactosylated Chitosan-Functionalized Mesoporous Silica Nanoparticle Loading by Calcium Leucovorin for Colon Cancer Cell-Targeted Drug Delivery. Molecules, 23(12), 3082. https://doi.org/10.3390/molecules23123082