Do Osmolytes Impact the Structure and Dynamics of Myoglobin?
Abstract
:1. Introduction
2. Results and Discussion
2.1. UV-vis Spectroscopy
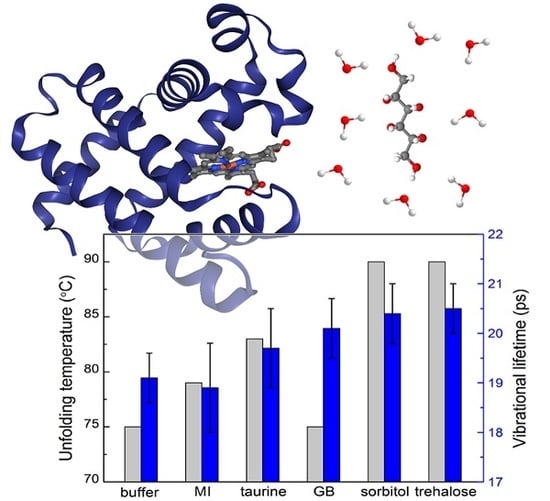
2.2. FTIR Spectroscopy
2.3. Polarization-Controlled IR Pump-Probe Spectroscopy
2.4. Circular Dichroism Spectroscopy
3. Materials and Methods
3.1. Materials
3.2. UV-vis Spectroscopy
3.3. FTIR and IR Pump−Probe Spectroscopy
3.4. Circular Dichroism Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Parker, M.W. Protein structure from X-ray diffraction. J. Biol. Phys. 2003, 29, 341–362. [Google Scholar] [CrossRef]
- von Dreele, R.B. Protein crystal structure analysis from high-resolution X-ray powder-diffraction data. Macromol. Crystallogr. Pt. C 2003, 368, 254–267. [Google Scholar]
- Raman, S.; Lange, O.F.; Rossi, P.; Tyka, M.; Wang, X.; Aramini, J.; Liu, G.; Ramelot, T.A.; Eletsky, A.; Szyperski, T.; et al. NMR structure determination for larger proteins using backbone-only data. Science 2010, 327, 1014–1018. [Google Scholar] [CrossRef] [PubMed]
- Sakakibara, D.; Sasaki, A.; Ikeya, T.; Hamatsu, J.; Hanashima, T.; Mishima, M.; Yoshimasu, M.; Hayashi, N.; Mikawa, T.; Wälchli, M.; et al. Protein structure determination in living cells by in-cell NMR spectroscopy. Nature 2009, 458, 102–105. [Google Scholar] [CrossRef] [PubMed]
- Müntener, T.; Häussinger, D.; Selenko, P.; Theillet, F.X. In-cell protein structures from 2D NMR experiments. J. Phys. Chem. Lett. 2016, 7, 2821–2825. [Google Scholar] [CrossRef]
- Day, C. Neutron diffraction overcomes flux limits to resolve a large protein structure. Phys. Today 2003, 56, 17–19. [Google Scholar] [CrossRef]
- Michalet, X.; Weiss, S.; Jäger, M. Single-molecule fluorescence studies of protein folding and conformational dynamics. Chem. Rev. 2006, 106, 1785–1813. [Google Scholar] [CrossRef]
- Book, L.D.; Arnett, D.C.; Hu, H.B.; Scherer, N.F. Ultrafast pump−probe studies of excited-state charge-transfer dynamics in blue copper proteins. J. Phys. Chem. A 1998, 102, 4350–4359. [Google Scholar] [CrossRef]
- Hamm, P.; Lim, M.H.; Hochstrasser, R.M. Structure of the amide I band of peptides measured by femtosecond nonlinear-infrared spectroscopy. J. Phys. Chem. B 1998, 102, 6123–6138. [Google Scholar] [CrossRef]
- Fayer, M.D. Dynamics of liquids, molecules, and proteins measured with ultrafast 2D IR vibrational echo chemical exchange spectroscopy. Annu. Rev. Phys. Chem. 2009, 60, 21–38. [Google Scholar] [CrossRef]
- Maj, M.; Kwak, K.; Cho, M. Ultrafast structural fluctuations of myoglobin-bound thiocyanate and selenocyanate ions measured with two-dimensional infrared photon echo spectroscopy. ChemPhysChem 2016, 17, 921. [Google Scholar] [CrossRef] [PubMed]
- Massari, A.M.; Finkelstein, I.J.; Fayer, M.D. Dynamics of proteins encapsulated in silica sol−gel glasses studied with IR vibrational echo spectroscopy. J. Am. Chem. Soc. 2006, 128, 3990–3997. [Google Scholar] [CrossRef] [PubMed]
- Groot, M.L.; Breton, J.; van Wilderen, L.J.G.W.; Dekker, J.P.; van Grondelle, R. Femtosecond visible/visible and visible/mid-IR pump−probe study of the photosystem II core antenna complex CP47. J. Phys. Chem. B 2004, 108, 8001–8006. [Google Scholar] [CrossRef]
- Xie, A.H.; Kelemen, L.; Redlich, B.; van der Meer, L.; Austin, R. Picosecond visible/IR pump-probe dynamics of photoactive yellow protein. Nucl. Instrum. Methods Phys. Res. A 2004, 528, 605–608. [Google Scholar] [CrossRef]
- Kim, T.W.; Lee, J.H.; Choi, J.; Kim, K.H.; van Wilderen, L.J.; Guerin, L.; Kim, Y.; Jung, Y.O.; Yang, C.; Kim, J.; et al. Protein structural dynamics of photoactive yellow protein in solution revealed by pump-probe X-ray solution scattering. J. Am. Chem. Soc. 2012, 134, 3145–3153. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Muniyappan, S.; Oang, K.Y.; Kim, J.G.; Nozawa, S.; Sato, T.; Koshihara, S.Y.; Henning, R.; Kosheleva, I.; Ki, H.; et al. Direct observation of cooperative protein structural dynamics of homodimeric hemoglobin from 100 ps to 10 ms with pump-probe X-ray solution scattering. J. Am. Chem. Soc. 2012, 134, 7001–7008. [Google Scholar] [CrossRef] [PubMed]
- Lindorff-Larsen, K.; Trbovic, N.; Maragakis, P.; Piana, S.; Shaw, D.E. Structure and dynamics of an unfolded protein examined by molecular dynamics simulation. J. Am. Chem. Soc. 2012, 134, 3787–3791. [Google Scholar] [CrossRef]
- Maisuradze, G.G.; Senet, P.; Czaplewski, C.; Liwo, A.; Scheraga, H.A. Investigation of protein folding by coarse-grained molecular dynamics with the UNRES force field. J. Phys. Chem. A 2010, 114, 4471–4485. [Google Scholar] [CrossRef]
- Nerukh, D.; Okimoto, N.; Suenaga, A.; Taiji, M. Ligand diffusion on protein surface observed in molecular dynamics simulation. J. Phys. Chem. Lett. 2012, 3, 3476–3479. [Google Scholar] [CrossRef]
- Berg, J.M.; Tymoczko, J.L.; Stryer, L. Biochemistry; W.H. Freeman: New York, NY, USA, 2012. [Google Scholar]
- Springer, B.A.; Sligar, S.G.; Olson, J.S.; Phillips, G.N. Mechanisms of ligand recognition in myoglobin. Chem. Rev. 1994, 94, 699–714. [Google Scholar] [CrossRef]
- Quillin, M.L.; Arduini, R.M.; Olson, J.S.; Phillips, G.N. High-resolution crystal-structures of distal histidine mutants of sperm whale myoglobin. J. Mol. Biol. 1993, 234, 140–155. [Google Scholar] [CrossRef] [PubMed]
- Petrich, J.W.; Martin, J.L. Ultrafast absorption and raman-spectroscopy of hemeproteins. Chem. Phys. 1989, 131, 31–47. [Google Scholar] [CrossRef]
- Bolard, J.; Garnier, A. Circular-dichroism studies of myoglobin and cytochrome c derivatives. Biochim. Biophys. Acta 1972, 263, 535–549. [Google Scholar] [CrossRef]
- Lerch, M.T.; Horwitz, J.; McCoy, J.; Hubbell, W.L. Circular dichroism and site-directed spin labeling reveal structural and dynamical features of high-pressure states of myoglobin. Proc. Natl. Acad. Sci. USA 2013, 110, E4714–E4722. [Google Scholar] [CrossRef]
- Goldbeck, R.A.; Bhaskaran, S.; Ortega, C.; Mendoza, J.L.; Olson, J.S.; Soman, J.; Kliger, D.S.; Esquerra, R.M. Water and ligand entry in myoglobin: Assessing the speed and extent of heme pocket hydration after CO photodissociation. Proc. Natl. Acad. Sci. USA 2006, 103, 1254–1259. [Google Scholar] [CrossRef] [PubMed]
- Bowen, W.J. The absorption spectra and extinction coefficients of myoglobin. J. Biol. Chem. 1949, 179, 235–245. [Google Scholar] [PubMed]
- Oldfield, E.; Guo, K.; Augspurger, J.D.; Dykstra, C.E. A molecular-model for the major conformational substates in heme proteins. J. Am. Chem. Soc. 1991, 113, 7537–7541. [Google Scholar] [CrossRef]
- Augspurger, J.D.; Dykstra, C.E.; Oldfield, E. Correlation of C-13 and O-17 chemical-shifts and the vibrational frequency of electrically perturbed carbon-monoxide—A possible model for distal ligand effects in carbonmonoxyheme proteins. J. Am. Chem. Soc. 1991, 113, 2447–2451. [Google Scholar] [CrossRef]
- Ferrante, C.; Pontecorvo, E.; Cerullo, G.; Vos, M.H.; Scopigno, T. Direct observation of subpicosecond vibrational dynamics in photoexcited myoglobin. Nat. Chem. 2016, 8, 1137–1143. [Google Scholar] [CrossRef] [Green Version]
- Hill, J.R.; Tokmakoff, A.; Peterson, K.A.; Sauter, B.; Zimdars, D.; Dlott, D.D.; Fayer, M.D. Vibrational dynamics of carbon-monoxide at the active-site of myoglobin—Picosecond infrared free-electron laser pump-probe experiments. J. Phys. Chem. 1994, 98, 11213–11219. [Google Scholar] [CrossRef]
- Gibson, Q.H.; Olson, J.S.; McKinnie, R.E.; Rohlfs, R.J. A kinetic description of ligand binding to sperm whale myoglobin. J. Biol. Chem. 1986, 261, 228–239. [Google Scholar]
- Xie, A.H.; van Der Meer, L.; Hoff, W.; Austin, R.H. Long-lived amide I vibrational modes in myoglobin. Phys. Rev. Lett. 2000, 84, 5435–5438. [Google Scholar] [CrossRef] [PubMed]
- Maj, M.; Oh, Y.; Park, K.; Lee, J.; Kwak, K.W.; Cho, M. Vibrational dynamics of thiocyanate and selenocyanate bound to horse heart myoglobin. J. Chem. Phys. 2014, 140, 235104. [Google Scholar] [CrossRef] [PubMed]
- Thielges, M.C.; Axup, J.Y.; Wong, D.; Lee, H.S.; Chung, J.K.; Schultz, P.G.; Fayer, M.D. Two-dimensional IR spectroscopy of protein dynamics using two vibrational labels: A site-specific genetically encoded unnatural amino acid and an active site ligand. J. Phys. Chem. B 2011, 115, 11294–11304. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Jin, G.; Lim, M. Dynamics of geminate recombination of NO with myoglobin in aqueous solution probed by femtosecond mid-IR spectroscopy. J. Phys. Chem. B 2004, 108, 20366–20375. [Google Scholar] [CrossRef]
- Helbing, J.; Bonacina, L.; Pietri, R.; Bredenbeck, J.; Hamm, P.; van Mourik, F.; Chaussard, F.; Gonzalez-Gonzalez, A.; Chergui, M.; Ramos-Alvarez, C.; et al. Time-resolved visible and infrared study of the cyano complexes of myoglobin and of hemoglobin I from Lucina pectinata. Biophys. J. 2004, 87, 1881–1891. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Kwak, K.W.; Cho, M. Computational infrared and two-dimensional infrared photon echo spectroscopy of both wild-type and double mutant myoglobin-co proteins. J. Phys. Chem. B 2013, 117, 15462–15478. [Google Scholar] [CrossRef]
- Bagchi, S.; Nebgen, B.T.; Loring, R.F.; Fayer, M.D. Dynamics of a myoglobin mutant enzyme: 2D IR vibrational echo experiments and simulations. J. Am. Chem. Soc. 2010, 132, 18367–18376. [Google Scholar] [CrossRef]
- Bolen, D.W.; Baskakov, I.V. The osmophobic effect: Natural selection of a thermodynamic force in protein folding. J. Mol. Biol. 2001, 310, 955–963. [Google Scholar] [CrossRef]
- Yancey, P.H.; Clark, M.E.; Hand, S.C.; Bowlus, R.D.; Somero, G.N. Living with water-stress-evolution of osmolyte systems. Science 1982, 217, 1214–1222. [Google Scholar] [CrossRef]
- Kumar, R. Role of naturally occurring osmolytes in protein folding and stability. Arch. Biochem. Biophys. 2009, 491, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.M. Osmosensing by bacteria: Signals and membrane-based sensors. Microbiol. Mol. Biol. Rev. 1999, 63, 230–262. [Google Scholar] [PubMed]
- Burg, M.B.; Ferraris, J.D. Intracellular organic osmolytes: Function and regulation. J. Biol. Chem. 2008, 283, 7309–7313. [Google Scholar] [CrossRef] [PubMed]
- Yancey, P.H. Water stress, osmolytes and proteins. Amer. Zool. 2001, 41, 699–709. [Google Scholar] [CrossRef]
- Auton, M.; Baskakov, I.; Bolen, C.L.; Bolen, D.W. On identifying the fundamental forces of osmolyte-induced protein stability. Biophys. J. 2001, 80, 558a. [Google Scholar]
- Bolen, D.W. Effects of naturally occurring osmolytes on protein stability and solubility: Issues important in protein crystallization. Methods 2004, 34, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.Y.; Pettitt, B.M.; Roesgen, J. Osmolyte solutions and protein folding. F1000 Biol. Rep. 2009, 1, 41. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Barkhordarian, H.; Emadi, S.; Park, C.B.; Sierks, M.R. Trehalose differentially inhibits aggregation and neurotoxicity of beta-amyloid 40 and 42. Neurobiol. Dis. 2005, 20, 74–81. [Google Scholar] [CrossRef]
- Xie, G.; Timasheff, S.N. The thermodynamic mechanism of protein stabilization by trehalose. Biophys. Chem. 1997, 64, 25–43. [Google Scholar] [CrossRef]
- Corradini, D.; Strekalova, E.G.; Stanley, H.E.; Gallo, P. microscopic mechanism of protein cryopreservation in an aqueous solution with trehalose. Sci. Rep. 2013, 3, 1218. [Google Scholar] [CrossRef]
- Singer, M.A.; Lindquist, S. Multiple effects of trehalose on protein folding in vitro and in vivo. Mol. Cell. 1998, 1, 639–648. [Google Scholar] [CrossRef]
- Chang, L.L.; Shepherd, D.; Sun, J.; Tang, X.C.; Pikal, M.J. Effect of sorbitol and residual moisture on the stability of lyophilized antibodies: Implications for the mechanism of protein stabilization in the solid state. J. Pharm. Sci. 2005, 94, 1445–1455. [Google Scholar] [CrossRef] [PubMed]
- Barbiroli, A.; Marengo, M.; Fessas, D.; Ragg, E.; Renzetti, S.; Bonomi, F.; Iametti, S. Stabilization of β-lactoglobulin by polyols and sugars against temperature-induced denaturation involves diverse and specific structural regions of the protein. Food Chem. 2017, 234, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Clegg, J.S.; Seitz, P.; Seitz, W.; Hazlewood, C.F. Cellular responses to extreme water loss: The water-replacement hypothesis. Cryobiology 1982, 19, 306–316. [Google Scholar] [CrossRef]
- Crowe, J.H.; Clegg, J.S.; Crowe, L.M. Anhydrobiosis: The water replacement hypothesis. In The Properties of Water in Foods ISOPOW 6; Reid, D.S., Ed.; Springer: Boston, MA, USA, 1998; pp. 440–455. ISBN 978-1-4613-0311-4. [Google Scholar]
- Golovina, E.A.; Golovin, A.V.; Hoekstra, F.A.; Faller, R. Water replacement hypothesis in atomic detail—Factors determining the structure of dehydrated bilayer stacks. Biophys. J. 2009, 97, 490–499. [Google Scholar] [CrossRef]
- Koster, K.L.; Webb, M.S.; Bryant, G.; Lynch, D.V. Interactions between soluble sugars and POPC (1-palmitoyl-2-oleoylphosphatidylcholine) during dehydration: Vitrification of sugars alters the phase behavior of the phospholipid. BBA-Biomembranes 1994, 1193, 143–150. [Google Scholar] [CrossRef]
- Willick, G.E.; Schonbaum, G.R.; Kay, C.M. Circular dichroism and absorption spectra of horse radish peroxidase and sperm whale myoglobin in soret region. Biochemistry 1969, 8, 3729–3734. [Google Scholar] [CrossRef]
- Denisov, V.P.; Halle, B. Protein hydration dynamics in aqueous solution. Faraday Discuss. 1996, 103, 227–244. [Google Scholar] [CrossRef]
- Park, S.; Saven, J.G. Statistical and molecular dynamics studies of buried waters in globular proteins. Proteins: Struct. Funct. Bioinf. 2005, 60, 450–463. [Google Scholar] [CrossRef]
- Mao, Y.; Ratner, M.A.; Jarrold, M.F. One water molecule stiffens a protein. J. Am. Chem. Soc. 2000, 122, 2950–2951. [Google Scholar] [CrossRef]
- Esquerra, R.M.; López-Peña, I.; Tipgunlakant, P.; Birukou, I.; Nguyen, R.L.; Soman, J.; Olson, J.S.; Kliger, D.S.; Goldbeck, R.A. Kinetic spectroscopy of heme hydration and ligand binding in myoglobin and isolated hemoglobin chains: An optical window into heme pocket water dynamics. Phys. Chem. Chem. Phys. 2010, 12, 10270–10278. [Google Scholar] [CrossRef] [PubMed]
- Olson, J.S.; Phillips, G.N. Kinetic pathways and barriers for ligand binding to myoglobin. J. Biol. Chem. 1996, 271, 17593–17596. [Google Scholar] [CrossRef] [PubMed]
- Phillips, G.N.; Pettitt, B.M. Structure and dynamics of the water around myoglobin. Protein Sci. 1995, 4, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Slama, I.; Abdelly, C.; Bouchereau, A.; Flowers, T.; Savouré, A. Diversity, distribution and roles of osmoprotective compounds accumulated in halophytes under abiotic stress. Ann. Bot. 2015, 115, 433–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moriyama, Y.; Takeda, K. Critical temperature of secondary structural change of myoglobin in thermal denaturation up to 130 °C and effect of sodium dodecyl sulfate on the change. J. Phys. Chem. B 2010, 114, 2430–2434. [Google Scholar] [CrossRef] [PubMed]
- Moilanen, D.E.; Piletic, I.R.; Fayer, M.D. Water dynamics in nafion fuel cell membranes: The effects of confinement and structural changes on the hydrogen bond network. J. Phys. Chem. C 2007, 111, 8884–8891. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |







| COMb | COMb + MI 0.9 M | COMb + Taurine 0.9 M | COMb + GB 3 M | COMb + Trehalose 1.5 M | COMb + Sorbitol 3 M | ||
|---|---|---|---|---|---|---|---|
| ωcenter (cm−1) | A1 | 1943.0 | 1943.8 | 1943.8 | 1943.9 | 1944.0 | 1944.6 |
| A3 | 1930.5 | 1931.3 | 1933.2 | 1935.6 | 1935.2 | 1933.9 | |
| A0 | 1967.6 | 1963.2 | 1967.3 | 1967.2 | 1966.9 | 1965.1 | |
| T1 (ps) | 19.1 ± 0.5 | 18.9 ± 0.9 | 19.7 ± 0.8 | 20.1 ± 0.6 | 20.5 ± 0.5 | 20.4 ± 0.6 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kossowska, D.; Kwak, K.; Cho, M. Do Osmolytes Impact the Structure and Dynamics of Myoglobin? Molecules 2018, 23, 3189. https://doi.org/10.3390/molecules23123189
Kossowska D, Kwak K, Cho M. Do Osmolytes Impact the Structure and Dynamics of Myoglobin? Molecules. 2018; 23(12):3189. https://doi.org/10.3390/molecules23123189
Chicago/Turabian StyleKossowska, Dorota, Kyungwon Kwak, and Minhaeng Cho. 2018. "Do Osmolytes Impact the Structure and Dynamics of Myoglobin?" Molecules 23, no. 12: 3189. https://doi.org/10.3390/molecules23123189
APA StyleKossowska, D., Kwak, K., & Cho, M. (2018). Do Osmolytes Impact the Structure and Dynamics of Myoglobin? Molecules, 23(12), 3189. https://doi.org/10.3390/molecules23123189