High-Frequency Heating Extraction Method for Sensitive Drug Analysis in Human Nails
Abstract
:1. Introduction
2. Results and Discussion
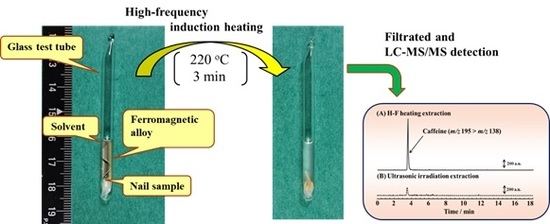
2.1. Features of H-F Heating Extraction
2.2. Optimization of H-F Heating Extraction for Nail Analysis
2.3. Application of H-F Heating Extraction to Nails from a Hypertension Patient
3. Materials and Methods
3.1. Chemicals
3.2. Apparatus
3.3. Sample Preparation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ribeiro, C.; Santos, C.; Gonçalves, V.; Ramos, A.; Afonso, C.; Tiritan, E.M. Chiral Drug Analysis in Forensic Chemistry: An Overview. Molecules 2018, 23, 262. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, H.; Zhu, H.; Yang, N.; Zhou, B.; Wang, C.; Li, P.; Liu, J. Pharmacokinetic and Metabolism Studies of 12-Riboside-Pseudoginsengenin DQ by UPLC-MS/MS and UPLC-QTOF-MSE. Molecules 2018, 23, 2499. [Google Scholar] [CrossRef] [PubMed]
- René, B.; Patrick, B.; Jean-Francois, B.; Pierre, D.; Gilles, P.; Éric, G.; Normand, F. New approach for the determination of ortho-phenylphenol exposure by measurement of sulfate and glucuronide conjugates in urine using liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2018, 410, 7275–7284. [Google Scholar]
- Takahashi, F.; Nitta, S.; Shimizu, R.; Jin, J. Electrochemiluminescence and voltammetry of tris(2,2′-bipyridine)ruthenium (II) with amphetamine-type stimulants as coreactants: An application to the discrimination of methamphetamine. Forensic Toxicol. 2018, 36, 185–191. [Google Scholar] [CrossRef]
- Tina, MB.; Franziska, G.; Clarissa, D.V.; Mathias, H.; Markus, R.B.; Thomas, K. Systematic investigations of endogenous cortisol and cortisone in nails by LC-MS/MS and correlation to hair. Anal. Bioanal. Chem. 2018, 410, 4895–4903. [Google Scholar]
- Kintz, P.; Spiehler, V.; Negrusz, A. Alternative specimens. In Clarke’s Analytical Forensic Toxicology; Jickells, S., Negrusz, A., Eds.; Pharmaceutical Press: London, UK, 2008; Volume 6, pp. 153–190. [Google Scholar]
- Suzuki, O.; Hattori, H.; Asano, M. Detection of methamphetamine and amphetamine in a single human hair by gas chromatography/chemical ionization mass spectrometry. J. Forensic Sci. 1984, 29, 611–617. [Google Scholar] [CrossRef]
- Nakahara, Y.; Takahashi, K.; Takeda, Y.; Konuma, K.; Fukui, S.; Tokui, T. Hair analysis for drug-abuse. 2. Hair analysis for monitoring of methamphetamine abuse by isotope-dilution gas-chromatography mass-spectrometry. Forensic Sci. Int. 1990, 46, 243–254. [Google Scholar] [CrossRef]
- Zhu, K.Y.; Leung, K.W.; Ting, A.K.; Wong, Z.C.; Ng, W.Y.; Choi, R.C.; Dong, T.T.; Wang, T.; Lau, D.T.; Tsim, K.W. Microfluidic chip based nano liquid chromatography coupled to tandem mass spectrometry for the determination of abused drugs and metabolites in human hair. Anal. Bioanal. Chem. 2012, 402, 2805–2815. [Google Scholar] [CrossRef]
- Phinney, K.W.; Sander, L.C. Liquid chromatographic method for the determination of enantiomeric composition of amphetamine and methamphetamine in hair samples. Anal. Bioanal. Chem. 2004, 378, 144–149. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Y. Matrix-assisted laser desorption/ionization mass spectrometric imaging for the rapid segmental analysis of methamphetamine in a single hair using umbelliferone as a matrix. Anal. Chim. Acta. 2017, 975, 42–51. [Google Scholar] [CrossRef]
- Miguez-Framil, M.; Moreda-Pineiro, A.; Bermejo-Barrera, P.; Cocho, J.A.; Tabernero, M.J.; Bermejo, A.M. Electrospray ionization tandem mass spectrometry for the simultaneous determination of opiates and cocaine in human hair. Anal. Chim. Acta. 2011, 704, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Emidio, E.S.; Prata, V.D.; Dorea, H.S. Validation of an analytical method for analysis of cannabinoids in hair by headspace solid-phase microextraction and gas chromatography-ion trap tandem mass spectrometry. Anal. Chim. Acta. 2010, 670, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Moriya, F. Alternative Specimens. In Handbook of Practical Analysis of Drugs and Poisons in Human Specimens—Chromatographic Methods; Jiho: Tokyo, Japan, 2002; Volume 1–2, pp. 8–14. [Google Scholar]
- Cappelle, D.; Doncker, D.M.; Gys, C.; Krysiak, K.; Keukeleire, D.S.; Maho, W.; Crunelle, C.L.; Dom, G.; Covaci, A.; Van-Nuijs, A.; et al. A straightforward, validated liquid chromatography coupled to tandem mass spectrometry method for the simultaneous detection of nine drugs of abuse and their metabolites in hair and nails. Anal. Chim. Acta. 2017, 960, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Busardo, F.P.; Gottardi, M.; Tini, A.; Mortali, C.; Giorgetti, R.; Pichini, R. Ultra-High-Performance Liquid Chromatography Tandem Mass Spectrometry Assay for Determination of Endogenous GHB and GHB-Glucuronide in Nails. Molecules 2018, 23, 2686. [Google Scholar] [CrossRef] [PubMed]
- Solimini, R.; Minutillo, A.; Kyriakou, C.; Pichini, S.; Pacifici, R.; Busardo, F.P. Nails in Forensic Toxicology: An Update. Curr. Pharm. Des. 2017, 23, 5468–5479. [Google Scholar] [CrossRef] [PubMed]
- Palmeri, A.; Pichini, S.; Pacifici, R.; Zuccaro, P.; Lopez, A. Drugs in nails: Physiology, pharmacokinetics and forensic toxicology. Clin. Pharmacokinet. 2000, 38, 95–110. [Google Scholar] [CrossRef] [PubMed]
- Simon, W.; Kriemler, P.; Voelimin, J.A.; Steiner, H. Elucidation of the structure of organic compound by thermal fragmentation. J. Gas Chromatogr. 1967, 5, 53–57. [Google Scholar] [CrossRef]
- Tsuge, S.; Ohtani, H.; Watanabe, C. Pyrolysis-GC/MS Data Book of Synthetic Polymers: Pyrograms, Thermograms and MS of Pyrolyzates; Elsevier: Amsterdam, the Netherlands, 2011. [Google Scholar]
- Michael, N.L. Pyrolysis and GC in Polymer Analysis; Marcel Dekker: New York, NY, USA, 1985. [Google Scholar]
- Ludovic, H.; Charlotte, H.; Béatrice, B.; Maria, K.; Rachid, A.; Anne-Laure, C.; Michel, L.; Ika, P.P.; Philippe, S.; Alexandre, D.; et al. Optimization, performance, and application of a pyrolysis-GC/MS method for the identification of microplastics. Anal. Bioanal. Chem. 2018, 410, 6663–6676. [Google Scholar]
- Kurihara, K.; Tsuchiya, F.; Takada, K.; Shoji, T. Pretreatment of analytical samples with high-frequency heating. DIC Tech. Rev. 2001, 7, 21–28. [Google Scholar]
- Kurihara, K.; Tanoue, F. Identification of fatty acid composing metal salt of it in resins by alcohol added thermal extraction with high-frequency heating. Bunseki Kagaku 2000, 49, 265–267. [Google Scholar] [CrossRef]
- Kurihara, K.; Tanoue, F. Qualitative analysis of a hindered phenol-type antioxidant by alcohol added thermal extraction with high-frequency heater, analyzing with GC/MS. Bunseki Kagaku 2000, 49, 205–208. [Google Scholar] [CrossRef]
- Kurihara, K.; Tanoue, F. Identification of polyester resin components by alcohol added thermal extraction with high-frequency heating. Bunseki Kagaku 2000, 49, 269–271. [Google Scholar] [CrossRef]
- Nakamura, M.; Tsuchiya, F.; Kurihara, K.; Takahashi, M. Quick molecular weight determination of polyester-polyurethane soft blocks with phenylisocyanate using high-frequency heating technique. Bunseki Kagaku 2007, 56, 237–240. [Google Scholar] [CrossRef]
- Takahashi, F.; Masaru, K.; Atsushi, K.; Kanya, K. Development of high-frequency heating extraction for drug analysis of human nails. Jpn. J. Forensic Sci. Tech. 2015, 20, 103–112. [Google Scholar] [CrossRef] [Green Version]
- Krumbiegel, F.; Hastedt, M.; Tsokos, M. Nails are a potential alternative matrix to hair for drug analysis in general unknown screenings by liquid-chromatography quadrupole time-of-flight mass spectrometry. Forensic Sci. Med. Path. 2014, 10, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Fujii, T.; Takashima, Y.; Takayama, S.; Ito, Y.; Kawasoe, T. Effect of heat treatment of human hair keratin film. Jpn. J. Cosmetic Sci. Soc. 2013, 37, 165–170. [Google Scholar]
- Nakamura, A.; Arimoto, M.; Takeuchi, K.; Fujii, T. A rapid extraction procedure of human hair proteins and identification of phosphorylated species. Biol. Pharm. Bull. 2002, 25, 569–572. [Google Scholar] [CrossRef]
- Wesolowski, M.; Szynkaruk, P. Thermal decomposition of methylxanthines interpretation of the results by PCA. J. Therm. Anal. Cal. 2016, 948, 40–47. [Google Scholar]
- Kuwayama, K.; Miyaguchi, H.; Iwata, Y.; Kanamori, T.; Tsujikawa, K.; Yamamuro, T.; Segawa, H.; Inoue, H. Three-step drug extraction from a single sub-millimeter segment of hair and nail to determine the exact day of drug intake. Anal. Chim. Acta. 2016, 948, 40–47. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are no more available from the authors. They have been used for analysis. |








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, F.; Kobayashi, M.; Kobayashi, A.; Kobayashi, K.; Asamura, H. High-Frequency Heating Extraction Method for Sensitive Drug Analysis in Human Nails. Molecules 2018, 23, 3231. https://doi.org/10.3390/molecules23123231
Takahashi F, Kobayashi M, Kobayashi A, Kobayashi K, Asamura H. High-Frequency Heating Extraction Method for Sensitive Drug Analysis in Human Nails. Molecules. 2018; 23(12):3231. https://doi.org/10.3390/molecules23123231
Chicago/Turabian StyleTakahashi, Fumiki, Masaru Kobayashi, Atsushi Kobayashi, Kanya Kobayashi, and Hideki Asamura. 2018. "High-Frequency Heating Extraction Method for Sensitive Drug Analysis in Human Nails" Molecules 23, no. 12: 3231. https://doi.org/10.3390/molecules23123231
APA StyleTakahashi, F., Kobayashi, M., Kobayashi, A., Kobayashi, K., & Asamura, H. (2018). High-Frequency Heating Extraction Method for Sensitive Drug Analysis in Human Nails. Molecules, 23(12), 3231. https://doi.org/10.3390/molecules23123231