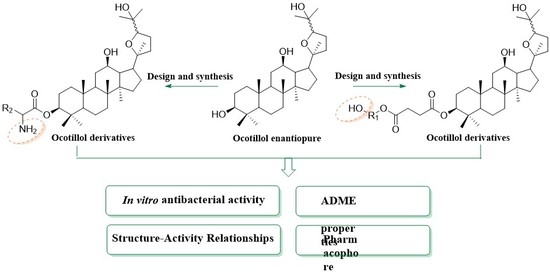
3.3 General Procedure for the Synthesis of Compounds 7–14
Diols (0.071 mmol) was added to a solution of
5 or
6 (0.035 mmol) in dry dichloromethane (10 mL), then DMAP (0.05 mmol) and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDCI) (0.05 mmol) were added. After stirring at room temperature for 4 h, the reaction mixture was washed with 10% hydrochloric acid, water and saturated brine, dried over anhydrous sodium sulfate and concentrated. The organic mixture was purified by column chromatography over silica gel (petroleum ether: acetone = 12:1–8:1).
1H-NMR,
13C-NMR and HR-MS spectra of compounds are shown in
Supplementary Materials.
4-[(20S,24R)-Epoxy-12β,25-diol-dammarane-3β-O]-4-oxo-butyryloxyethanol (7). White solid (yield 83.8%); m.p. 39–40 °C; HR-MS (HESI) m/z calcd. for C36H60O8 [M + H]+: 621.43610, found: 621.43286; 1H-NMR (CDCl3 400 MHz) δ (ppm): 0.83 (6H, s, -CH3), 0.84 (6H, s, -CH3), 0.87 (3H, s, -CH3), 0.89 (3H, s, -CH3), 0.97 (3H, s, -CH3), 1.09 (3H, s, -CH3), 1.25 (3H, s, -CH3), 1.27 (3H, s, -CH3), 2.66 (4H, s, -CH2CH2-), 3.47 (1H, td, J = 10.4 Hz, J = 4.5 Hz), 3.80 (1H, td, J = 8.6 Hz, J = 6.8 Hz; 2H, t, J = 4.5 Hz), 4.23 (2H, t, J = 4.5 Hz), 4.47 (1H, dd, J = 8.6 Hz, J = 7.7 Hz); 13C-NMR (CDCl3, 100 MHz) δ (ppm): 26.09, 27.58, 27.90, 28.56, 29.30, 29.34, 29.63, 29.67, 31.17, 31.28, 31.90, 32.58, 34.69, 37.02, 37.88, 38.56, 39.72, 47.91, 49.34, 50.36, 51.97, 55.99, 61.04, 66.30, 70.09, 70.91, 81.40, 85.38, 86.49, 172.23, 172.53.
4-[(20S,24S)-Epoxy-12β,25-diol-dammarane-3β-O]-4-oxo-butyryloxyethanol (8). White solid (yield 84.9%); m.p. 73–76 °C; HR-MS (HESI) m/z calcd. for C36H60O8 [M + H]+: 621.43610, found: 621.43457; 1H-NMR (CDCl3, 400 MHz) δ (ppm): 0.84 (3H, s, -CH3), 0.85 (6H, s, -CH3), 0.90 (3H, s, -CH3), 1.01 (3H, s, -CH3), 1.10 (3H, s, -CH3), 1.22 (3H, s, -CH3), 1.27 (3H, s, -CH3), 2.66 (4H, s, -CH2CH2-), 3.49 (1H, td, J = 10.3 Hz, J = 4.7 Hz), 3.79 (2H, t, J = 4.7 Hz), 3.85 (1H, dd, J = 10.7 Hz, J = 5.3 Hz), 4.22 (2H, t, J = 4.5 Hz), 4.48 (1H, dd, J = 9.2 Hz, J = 7.2 Hz), 5.7 (1H, s); 13C-NMR (CDCl3, 100 MHz) δ (ppm): 24.15, 25.01, 27.88, 27.91, 28.47, 28.81, 29.25, 29.58, 31.56, 31.58, 32.14, 34.60, 37.00, 37.85, 38.49, 39.68, 48.72, 48.83, 50.05, 52.09, 55.92, 60.90, 66.26, 70.02, 70.42, 81.35, 87.09, 87.32, 172.17, 172.5.
4-[(20S,24R)-Epoxy-12β,25-diol-dammarane-3β-O]-4-oxo-butyryloxybutanol (9). White solid (yield 84.8%); m.p. 54–57 °C; HR-MS (HESI) m/z calcd. for C38H64O8 [M + H]+: 649.46740, found: 649.46625; 1H-NMR (CDCl3, 400 MHz) δ (ppm): 0.84 (6H, s, -CH3), 0.87 (3H, s, -CH3), 0.89 (3H, s, -CH3), 0.98 (3H, s, -CH3), 1.09 (3H, s, -CH3), 1.26 (3H, s, -CH3), 1.27 (3H, s, -CH3), 2.62 (4H, s, -CH2CH2-), 3.47 (1H, td, J = 10.2 Hz, J = 4.4 Hz), 3.66 (2H, t, J = 6.4 Hz), 3.82 (1H, td, J = 8.6 Hz, J = 6.8 Hz), 4.11 (2H, t, J = 6.4 Hz), 4.46 (1H, dd, J = 10.3 Hz, J = 6.2 Hz); 13C-NMR (CDCl3, 100 MHz) δ (ppm): 26.13, 27.58, 27.90, 27.93, 28.58, 29.09, 29.26, 29.56, 29.68, 31.19, 31.33, 32.60, 34.74, 37.06, 37.90, 38.60, 39.75, 47.95, 49.39, 50.40, 52.00, 56.06, 62.33, 64.50, 70.09, 70.93, 81.21, 85.41, 86.51, 171.98, 172.36.
4-[(20S,24S)-Epoxy-12β,25-diol-dammarane-3β-O]-4-oxo-butyryloxybutanol (10). White solid (yield 81%); m.p. 57–59 °C; HR-MS (HESI) m/z calcd. for C38H64O8 [M + H]+: 649.46740, found: 649.46637; 1H-NMR (CDCl3, 400 MHz) δ (ppm): 0.85 (6H, s, -CH3), 0. 90 (3H, s, -CH3), 0.98 (3H, s, -CH3), 1.01 (3H, s, -CH3), 1.10 (3H, s, -CH3), 1.22 (3H, s, -CH3), 1.25 (3H, s, -CH3), 1.27 (3H, s, -CH3), 2.62 (4H, s, -CH2CH2-), 3.49 (1H, td, J = 10.2 Hz, J = 4.6 Hz), 3.64 (2H, t, J = 6.4 Hz) 3.85 (1H, td, J = 10.7 Hz, J = 5.3 Hz), 4.11 (2H, t, J = 6.3 Hz), 4.47 (1H, dd, J = 10.3 Hz, J = 6.0 Hz); 13C-NMR (CDCl3, 100 MHz) δ (ppm): 28.46, 28.80, 29.01, 29.19, 29.29, 29.49, 29.62, 29.95, 31.54, 31.58, 31.85, 32.13, 32.69, 34.60, 36.99, 37.36, 37.85, 38.48, 39.67, 48.71, 48.82, 50.04, 52.08, 55.93, 62.13, 64.47, 69.99, 70.40, 81.14, 87.07, 87.31, 171.92, 172.32, 171.32, 172.32.
4-[(20S,24R)-Epoxy-12β,25-diol-dammarane-3β-O]-4-oxo-butyryloxybutynol (11). White solid (yield 85.6%); m.p. 40 °C; HR-MS (HESI) m/z calcd. for C38H60O8 [M + H]+: 645.43610, found: 645.43481; 1H-NMR (CDCl3, 400 MHz) δ (ppm): 0.84 (3H, s, -CH3), 0.87 (3H, s, -CH3), 0.89 (3H, s, -CH3), 0.97 (3H, s, -CH3), 1.10 (3H, s, -CH3), 1.25 (3H, s, -CH3), 1.26 (3H, s, -CH3), 1.27 (3H, s, -CH3), 2.62 (4H, m, -CH2CH2-), 3.47 (1H, td, J = 10.4 Hz, J = 4.5 Hz), 3.82 (1H, td), 4.29 (2H, s), 4.46 (1H, dd, J = 10.1 Hz, J = 6.5 Hz), 4.73 (2H, s); 13C-NMR (CDCl3, 100 MHz) δ (ppm): 26.10, 27.57, 27.88, 27.92, 28.56, 29.04, 29.38, 29.67, 31.18, 31.31, 31.90, 32.59, 34.72, 37.05, 37.88, 38.58, 39.74, 47.93, 49.36, 50.38, 50.97, 51.99, 52.45, 56.04, 70.11, 70.92, 79.59, 81.33, 85.13, 85.39, 86.50, 171.62, 171.74.
4-[(20S,24S)-Epoxy-12β,25-diol-dammarane-3β-O]-4-oxo-butyryloxybutynol (12). White solid (yield 80.7%); m.p. 64 °C; HR-MS (HESI) m/z calcd. for C38H60O8 [M + H]+: 645.43610, found: 645.43494; 1H-NMR (CDCl3, 400 MHz) δ (ppm): 0.85 (6H, s, -CH3), 0.90 (3H, s, -CH3), 1.01 (3H, s, -CH3), 1.10 (3H, s, -CH3), 1.23 (3H, s, -CH3), 1.25 (3H, s, -CH3), 1.27 (3H, s, -CH3), 2.62 (4H, m, -CH2CH2-), 3.49 (1H, td, J = 10.3 Hz, J = 4.7 Hz), 3.82 (1H, td, J = 10.8 Hz, J = 5.3 Hz), 4.28 (2H, t, J = 1.7 Hz), 4.48 (1H, dd, J = 10.3 Hz, J = 6.1 Hz), 4.73 (2H, t, J = 3.4 Hz), 5.8 (2H, s); 13C-NMR (CDCl3, 100 MHz) δ (ppm): 27.91, 27.97, 28.50, 28.84, 29.02, 29.36, 29.66, 31.58, 31.62, 31.89, 32.18, 34.63, 37.04, 37.87, 38.51, 39.71, 48.75, 48.85, 50.08, 50.92, 52.12, 52.46, 55.97, 70.04, 70.45, 79.51, 81.32, 85.18, 87.13, 87.35, 128.80, 171.64, 171.73.
4-[(20S,24R)-Epoxy-12β,25-diol-dammarane-3β-O]-4-oxo-butyryloxy phenol (13). White solid (yield 83%); m.p. 68 °C; HR-MS (HESI) m/z calcd. for C40H60O8 [M + H]+: 669.43610, found: 669.43536; 1H-NMR (CDCl3, 400 MHz) δ (ppm): 0.84 (3H, s, -CH3), 0.85 (3H, s, -CH3), 0.89 (3H, s, -CH3), 0.97 (3H, s, -CH3), 1.10 (3H, s, -CH3), 1.25 (3H, s, -CH3), 1.26 (3H, s, -CH3), 1.28 (3H, s, -CH3), 2.71 (2H, t, -CH2-), 2.83, (2H, t, -CH2-), 3.51 (1H, td, J = 8.4 Hz), 3.82 (1H, td, J = 6.8 Hz), 4.49 (1H, dd, J = 10.1 Hz, J =5.0 Hz), 6.78 (2H, Ph), 6.90 (2H, Ph); 13C-NMR (CDCl3, 100 MHz) δ (ppm): 26.06, 27.62, 27.90, 27.98, 28.58, 29.37, 29.52, 29.68, 30.02, 31.21, 31.31, 31.91, 32.62, 34.73, 37.04, 37.92, 38.58, 39.75, 47.95, 49.38, 50.41, 52.02, 56.05, 70.32, 71.01, 81.40, 85.38, 86.54, 115.92, 122.32, 143.95, 153.63, 171.32, 171.85.
4-[(20S,24S)-Epoxy-12β,25-diol-dammarane-3β-O]-4-oxo-butyryloxy phenol (14). White solid (yield 79%); m.p. 88 °C; HR-MS (HESI) m/z calcd. for C40H60O8 [M + H]+: 669.43610, found: 669.43536; 1H-NMR (CDCl3, 400 MHz) δ (ppm): 0.84 (3H, s, -CH3), 0.86 (3H, s, -CH3), 0.89 (3H, s, -CH3), 0.91 (3H, s, -CH3), 1.01 (3H, s, -CH3), 1.10 (3H, s, -CH3), 1.25 (3H, s, -CH3), 1.27 (3H, s, -CH3), 2.71 (2H, t, -CH2-), 2.84 (2H, t, -CH2-), 3.52 (1H, td, J = 8.4 Hz), 3.85 (1H, dd, J = 10.8 Hz, J = 5.3 Hz), 4.50 (1H, dd, J = 5.8 Hz, J = 10.6 Hz), 5.95 (H, s, OH), 6.17 (H, s, OH), 6.78 (2H, Ph), 6.90 (2H, Ph); 13C-NMR (CDCl3, 100 MHz) δ (ppm): 24.11, 25.02, 27.95, 27.97, 28.50, 28.82, 28.31, 29.46, 29.67, 31.54, 31.57, 32.19, 34.61, 37.01, 37.88, 38.48, 39.69, 48.68, 48.80, 50.06, 52.13, 55.94, 70.10, 70.58, 81.37, 87.18, 87.36, 115.92, 122.28, 143.78, 153.74, 171.41, 171.89.
3.4. General Procedure for the Synthesis of Compounds 15–26
To a solution of 3 or 4 in dry dichloromethane (10 mL), Boc-amino acids (0.21 mmol), DMAP (0.05 mmol) and EDCI (0.15 mmol) were added, and the mixture was stirred at room temperature for 7 h. The reaction mixture was washed with water and saturated brine, dried over anhydrous sodium sulfate, filtered, and concentrated. The organic mixture was purified by column chromatography over silica gel (trichloromethane:methanol = 250:1). The above intermediate (0.036 mmol) was dissolved in ethyl acetate-35% HCl (10 mL, 20:1), then stirred at 35 °C for 2 h. The reaction solution was washed with saturated sodium bicarbonate, water, and saturated brine in that order, dried over anhydrous sodium sulfate, filtered, and concentrated. The organic mixture was purified by column chromatography over silica gel (trichloromethane: methanol = 200:1) to provide compounds 15–20.
The intermediate of Boc-amino acids at C-3 was directly dissolved in dry dichloromethane (8 mL), trifluoroacetic acid (1 mL) was added, and the mixture was stirred at room temperature for 2 h. The reaction solution was washed with saturated sodium bicarbonate, water, and saturated brine in that order, dried over anhydrous sodium sulfate, filtered, and concentrated. Silica gel column chromatography (dichloromethane:methanol = 80:1–10:1) gave the desired product 21–26.
(20S,24R)-Epoxy-3β-O-(2-aminopropionyl)-dammarane-12β,25-diol (15). Light yellow solid (90.2%); m.p. 78–80 °C; HR-MS (HESI) m/z calcd. for C33H57NO5 [M + H]+: 548.4315, found: 548.4321; 1H-NMR (CDCl3, 300 MHz) δ (ppm): 0.77(3H, s, -CH3), 0.79 (3H, s, -CH3), 0.81 (3H, s, -CH3), 0.83 (3H, s, -CH3), 0.91 (3H, s, -CH3), 1.02 (3H, s, -CH3), 1.19 (3H, s, -CH3), 1.20 (3H, s, -CH3), 1.37 (3H, s, -CH3), 3.43 (1H, dt, J = 10.2 Hz, J = 4.7 Hz, -OCH-), 3.58 (1H, m, -NCH-), 3.78 (1H, dd, J = 10.8 Hz, J = 5.4 Hz, -OCH-), 4.45 (1H, dd, J = 10.4 Hz, J = 6.0 Hz, -OCH-); 13C-NMR (CDCl3, 75 MHz) δ (ppm): 15.38, 16.36, 16.48, 18.13, 18.17, 20.08, 23.68, 25.00, 26.13, 27.60, 27.91, 28.00, 28.59, 29.70, 31.19, 31.33, 32.61, 34.72, 37.06, 38.05, 38.56, 39.74, 47.94, 49.37, 50.39, 52.00, 56.01, 70.11, 70.92, 81.73, 85.41, 86.51, 175.21.
(20S,24S)-Epoxy-3β-O-(2-aminopropionyl)-dammarane-12β,25-diol (16). Light yellow solid (82.1%); m.p. 79–80 °C; HR-MS (HESI) m/z calcd. for C33H57NO5 [M + H]+: 548.4315, found: 548.4326; 1H-NMR (CDCl3, 300 MHz) δ (ppm): 0.85 (3H, s, -CH3), 0.87 (3H, s, -CH3), 0.92 (6H, s, -CH3), 1.02 (3H, s, -CH3), 1.11 (3H, s, -CH3), 1.23 (3H, s, -CH3), 1.28 (3H, s, -CH3), 1.40 (3H, s, -CH3), 3.53 (1H, dt, J = 10.2 Hz, J = 4.7 Hz, -OCH-), 3.58 (1H, m, -NCH-), 3.88 (1H, dd, J = 10.8 Hz, J = 5.4 Hz, -OCH-), 4.53 (1H, dd, J = 10.4 Hz, J = 6.0 Hz, -OCH-), 5.80 (1H, s, -OH); 13C-NMR (CDCl3, 75 MHz) δ (ppm): 15.45, 16.32, 16.50, 17.77, 18.14, 20.29, 23.70, 24.18, 25.08, 26.13, 27.60, 27.91, 28.53, 28.87, 31.60, 31.68, 32.20, 34.65, 37.07, 38.05, 38.52, 39.74, 48.78, 48.88, 50.11, 52.14, 55.97, 70.08, 70.46, 81.54, 87.13, 87.39, 175.23.
(20S,24R)-Epoxy-3β-O-[(2-amino-4-methyl)pentanoyl)-dammarane-12β,25-diol (17). Light yellow solid (76.2%); m.p. 79–81 °C; HR-MS (ESI) m/z calcd. for C36H63NO5 [M + H]+: 590.4784, found: 590.4779; 1H-NMR (CDCl3, 300 MHz) δ (ppm): 0.75 (3H, s, -CH3), 0.77 (3H, s, -CH3), 0.79 (3H, s, -CH3), 0.80 (3H, s, -CH3), 0.85 (3H, s, -CH3), 0.89 (3H, s, -CH3), 1.00 (3H, s, -CH3), 1.17 (3H, s, -CH3), 1.18 (3H, s, -CH3), 1.37 (3H, s, -CH3), 3.43 (1H, dt, J = 10.2 Hz, J = 4.7 Hz, -OCH-), 3.45 (1H, m, -NCH-), 3.74 (1H, dd, J = 10.8 Hz, J = 5.4 Hz, -OCH-), 4.45 (1H, dd, J = 10.4 Hz, J = 6.0 Hz, -OCH-); 13C-NMR (CDCl3, 75 MHz) δ (ppm): 15.38, 16.38, 16.55, 18.17, 20.08, 22.15, 23.67, 24.81, 25.00, 26.14, 27.60, 27.92, 28.09, 28.59, 29.70, 31.19, 31.33, 32.60, 34.74, 37.07, 37.96, 38.05, 38.53, 39.75, 43.75, 47.94, 49.36, 50.40, 52.01, 56.07, 70.11, 70.94, 81.73, 85.41, 86.51, 175.20.
(20S,24S)-Epoxy-3β-O-[(2-amino-4-methyl)pentanoyl)-dammarane-12β,25-diol (18). Light yellow solid (77.1%); m.p. 76–78 °C; HR-MS (ESI) m/z calcd. for C36H63NO5 [M + H]+: 590.4784, found: 590.4778; 1H-NMR (CDCl3, 300 MHz) δ (ppm): 0.79 (3H, s, -CH3), 0.80 (3H, s, -CH3), 0.85 (6H, s, -CH3), 0.88 (6H, s, -CH3), 0.94 (3H, s, -CH3), 1.04 (3H, s, -CH3), 1.16 (3H, s, -CH3), 1.21 (3H, s, -CH3), 3.43 (1H, dt, J = 10.2 Hz, J = 4.7 Hz, -OCH-), 3.45 (1H, m, -NCH-), 3.81 (1H, dd, J = 10.8 Hz, J = 5.4 Hz, -OCH-), 4.45 (1H, dd, J = 10.4 Hz, J = 6.0 Hz, -OCH-); 13C-NMR (CDCl3, 100 MHz) δ (ppm): 15.38, 16.38, 16.55, 18.17, 20.08, 22.15, 23.67, 24.81, 25.00, 26.14, 27.60, 27.92, 28.09, 28.59, 29.70, 31.19, 31.33, 32.60, 34.74, 37.07, 37.96, 38.05, 38.53, 39.75, 43.75, 47.94, 49.36, 50.40, 52.01, 56.07, 70.11, 70.94, 81.73, 87.10, 87.33, 175.23.
(20S,24R)-Epoxy-3β-O-[(2-amino-3-methyl)butanoyl)-dammarane-12β,25-diol (19). Light yellow solid (79.9%); m.p. 77–79 °C; HR-MS (ESI) m/z calcd. for C36H63NO5 [M + H]+: 576.4628, found: 576.4627; 1H-NMR (CDCl3, 400 MHz) δ (ppm): 0.87(6H, s, -CH3), 0.89 (6H, s, -CH3), 0.91 (6H, s, -CH3), 1.01 (3H, s, -CH3), 1.10 (3H, s, -CH3), 1.23 (3H, s, -CH3), 1.28 (3H, s, -CH3), 3.28 (1H, d, J = 4.5, -OCH-), 3.53 (1H, dt, J = 10.2 Hz, J = 4.7 Hz, -NCH-), 3.88 (1H, dd, J = 10.8 Hz, J = 5.4 Hz, -OCH-), 4.52 (1H, dd, J = 10.4 Hz, J = 6.0 Hz, -OCH-); 13C-NMR (CDCl3, 100 MHz) δ (ppm): 15.33, 16.38, 16.43, 18.12, 19.22, 20.22, 23.18, 23.63, 24.89, 26.08, 27.56, 27.87, 28.30, 28.53, 29.65, 31.14, 31.27, 32.57, 34.66, 37.00, 37.99, 38.51, 39.69, 47.88, 49.32, 50.34, 51.95, 52.64, 55.96, 70.08, 70.88, 81.53, 85.35, 86.46, 175.52.
(20S,24S)-Epoxy-3β-O-[(2-amino-3-methyl)butanoyl)-dammarane-12β,25-diol (20). Light yellow solid (82.2%); m.p. 79–81 °C; HR-MS (ESI) m/z calcd. for C36H63NO5 [M + H]+: 576.4628, found: 576.4636; 576.4636; 1H-NMR (CDCl3, 400 MHz) δ (ppm): 0.87(6H, s, -CH3), 0.90 (6H, s, -CH3), 1.01 (6H, s, -CH3), 1.10 (6H, s, -CH3), 1.23 (3H, s, -CH3), 1.28 (3H, s, -CH3), 3.28 (1H, d, J=4.5, -OCH-), 3.53 (1H, dt, J = 10.2 Hz, J = 4.7 Hz, -NCH-), 3.88 (1H, dd, J = 10.8, J = 5.4 Hz, -OCH-), 4.52 (1H, dd, J = 10.4 Hz, J = 6.0 Hz, -OCH-); 13C-NMR (CDCl3, 100 MHz) δ (ppm): 15.42, 16.28, 16.59, 16.69, 17.72, 18.14, 19.64, 23.75, 24.21, 25.00, 28.00, 28.03, 28.50, 28.84, 31.56, 31.64, 31.74, 32.16, 34.64, 37.02, 37.88, 38.50, 39.70, 48.76, 48.87, 50.08, 52.11, 55.98, 60.31, 69.98, 70.82, 81.28, 87.10, 87.33, 175.20.
(20S,24R)-Epoxy-3β-O-(2S-amino-phenylpropionyl)-dammarane-12β,25-diol (21). White solid (yield 45%); m.p. 230–233 °C; HR-MS (HESI) m/z calcd. for C39H61NO5 [M + H]+: 624.46225, found: 624.46115; 1H-NMR (CDCl3, 300 MHz) δ (ppm) 0.76 (3H, s, -CH3), 0.78 (3H, s, -CH3), 0.85 (3H, s, -CH3), 0.93 (3H, s, -CH3), 1.05 (3H, s, -CH3), 1.16 (3H, s, -CH3), 1.18 (3H, s, -CH3), 1.22 (3H, s, -CH3), 2.21–2.14 (1H, m, -CH2-), 2.74 (1H, dd, J = 13.3 Hz, 8.5 Hz, -CH2-), 3.10 (1H, dd, J = 13.5 Hz, 4.9 Hz, -CH2-), 3.46 (1H, td, J = 10.3 Hz, 4.6 Hz, -OCH-), 3.66 (1H, t, J = 10.4 Hz, -NCH-), 3.83 (1H, dd, J = 11.2 Hz, 5.4 Hz, -OCH-), 4.47 (1H, dd, J = 10.3 Hz, 5.9 Hz, -OCH-), 7.23–7.15 (5H, m, Ar-H). 3C-NMR (CDCl3, 100 MHz) δ (ppm) 13.81. 15.47, 16.44, 16.57, 18.23, 19.26, 23.72, 25.08, 26.21, 27.67, 27.99, 28.07, 28.67, 30.65, 31.28, 31.42, 32.69, 34.82, 37.14, 38.04, 38.68, 39.85, 48.04, 49.47, 50.50, 52.09, 56.14, 65.65, 71.02, 70.21, 81.93, 85.49, 86.59, 126.93, 128.69, 129.40, 137.25, 167.80.
(20S,24S)-Epoxy-3β-O-(2S-amino-phenylpropionyl)-dammarane-12β,25-diol (22). White solid (yield 43%); m.p. 236–240 °C; HR-MS (HESI) m/z calcd. for C39H61NO5 [M + H]+: 624.46225, found: 624.46149; 1H-NMR (CDCl3, 300 MHz) δ (ppm) 0.71 (3H, s, -CH3), 0.78 (3H, s, -CH3), 0.84 (3H, s, -CH3), 0.94 (3H, s, -CH3), 1.03 (3H, s, -CH3), 1.17 (3H, s, -CH3), 1.19 (3H, s, -CH3), 1.21 (3H, s, -CH3), 2.21–2.14 (1H, m, -CH2-), 2.73 (1H, dd, J = 13.4 Hz, 8.4 Hz, -CH2-), 3.09 (1H, dd, J = 13.4 Hz, 4.9 Hz, -CH2-), 3.46 (1H, td, J = 10.4 Hz, 4.7 Hz, -OCH-), 3.65 (1H, t, J = 10.0 Hz, -NCH-), 3.81 (1H, dd, J = 11.0 Hz, 5.6 Hz, -OCH-), 4.46 (1H, dd, J = 10.4 Hz, 5.8 Hz, -OCH-), 7.26–7.14 (5H, m, Ar-H).13C-NMR (CDCl3, 100 MHz) δ (ppm) 13.81. 15.55, 16.40, 16.59, 17.84, 19.26, 23.74, 24.27, 25.18, 28.08, 28.96, 30.65, 31.71, 32.29, 34.76, 37.16, 38.04, 38.64, 39.84, 48.89, 49.00, 50.22, 52.23, 56.10, 65.65, 70.18, 70.56, 81.96, 87.22, 87.48, 126,94, 128.70, 129.40, 137.21, 167.79.
(20S,24R)-Epoxy-3β-O-(2S-amino-3-mercaptopropanoyl)-dammarane-12β,25-diol (23). White solid (yield 39%); m.p. 235–237 °C; HR-MS (HESI) m/z calcd. for C41H62N2O5 [M + H]+: 663.47370, found: 663.47272; 1H-NMR (CDCl3, 300 MHz) δ (ppm) 0.78 (3H, s, -CH3); 0.84 (3H, s, -CH3), 0.87 (3H, s, -CH3), 0.90 (3H, s, -CH3), 0.98 (3H, s, -CH3), 1.09 (3H, s, -CH3), 1.27 (3H, s, -CH3), 1.28 (3H, s, -CH3), 2.19 (1H, t, J = 8.8 Hz, -CH2-), 2.95 (1H, dd, J = 14.3 Hz, 8.6 Hz, -CH2-), 3.35 (1H, dd, J = 14.3 Hz, 4.4 Hz, -CH2-), 3.52 (1H, dd, J = 10.4 Hz, 6.1 Hz, -OCH-), 3.85 (2H, t, J = 8.6 Hz, -OCH-), 4.52 (1H, t, J = 8.7 Hz, -OCH-), 5.61 (1H, s, Ar-H), 7.16 (2H, td, J = 14.9 Hz, 7.0 Hz, Ar-H), 7.36 (1H, d, J = 7.9 Hz, -NH-), 7.64 (1H, d, J = 7.6 Hz, Ar-H).13C-NMR (CDCl3, 100 MHz) δ (ppm) 15.47, 16.44, 16.59, 17.83, 18.24, 23.63, 24.25, 25.09, 26.18, 27.66, 27.99, 28.44, 29.78, 31.27, 32.69, 34.81, 37.13, 38.08, 39.84, 48.03, 49.45, 50.49, 52.10, 52.23, 56.13, 70.19, 70.26, 71.03, 82.42, 85.49, 86.60, 111.46, 118.67, 119.70, 122.36, 123.61, 127.30, 128.44, 129.45, 129.63, 136.21, 136.45, 170.97.
(20S,24S)-Epoxy-3β-O-(2S-amino-3-mercaptopropanoyl)-dammarane-12β,25-diol (24). White solid (yield 41%); m.p. 225–229 °C; HR-MS (HESI) m/z calcd. for C41H62N2O5 [M + H]+: 663.47370, found: 663.47284; 1H-NMR (CDCl3, 300 MHz) δ (ppm) 0.79 (3H, s, -CH3), 0.85 (3H, s, -CH3), 0.91 (3H, s, -CH3), 1.01 (3H, s, -CH3), 1.10 (3H, s, -CH3), 1.23 (3H, s, -CH3), 1.25 (3H, s, -CH3), 1.28 (3H, s, -CH3), 2.25 (1H, m, -CH2-), 2.95 (1H, dd, J = 14.2 Hz, 8.6 Hz, -CH2-), 3.35 (1H, dd, J = 14.3 Hz, 4.5 Hz, -CH2-), 3.53 (1H, td, J = 10.2 Hz, 5.6 Hz, -OCH-), 3.86 (2H, m, -OCH-), 4.53 (1H, t, J = 9.6 Hz, -OCH-), 5.76 (1H, s, Ar-H), 7.15 (2H, m, Ar-H), 7.36 (1H, d, J = 7.8 Hz, Ar-H), 7.64 (1H, d, J = 7.7Hz, -NH-).13C-NMR (CDCl3, 100 MHz) δ (ppm) 15.54, 16.42, 16.64, 17.86, 18.24, 23.76, 24.31, 25.14, 28.06, 28.14, 28.64, 28.97, 29.42, 29.80, 31.19, 31.70, 32.29, 34.75, 37.15, 38.06, 38.63, 39.83, 48.88, 48.98, 50.21, 52.24, 55.35, 56.09, 70.14, 70.56, 81.60, 87.24, 87.46, 111.33, 111.53, 118.88, 119.59, 122.26, 123.02, 127.49, 136.40, 174.92.
(20S,24R)-Epoxy-3β-O-(L-lysyl)-dammarane-12β,25-diol (25). White solid (yield 52%); m.p. 201–203 °C, HR-MS (HESI) m/z calcd. for C36H64N2O5 [M + H]+: 605.48880, found: 605.48920; 1H-NMR (CDCl3, 400 MHz) δ (ppm) 0.82 (3H, s, -CH3), 0.84 (3H, s, -CH3), 0.87 (3H, s, -CH3), 0.89 (3H, s, -CH3), 0.97 (3H, s, -CH3), 1.08 (3H, s, -CH3), 1.25 (3H, s, -CH3), 1.26 (3H, s, -CH3), 2.25 (1H, m, NCH2-), 2.95 (1H, dd, J = 14.2 Hz, 8.6 Hz, NCH2-), 3.36 (2H, dd, J = 12.0 Hz, 4.0 Hz, -NCH2-), 3.49 (2H, dt, J = 10.3 Hz, 4.5 Hz, -OCH-), 3.83 (1H, m, -OCH-), 4.49 (1H, t, J = 8.3 Hz, -OCH-).13C-NMR (CDCl3, 100 MHz) δ (ppm) 15.46. 16.43, 16.65, 18.22, 22.85, 23.80, 25.06, 26.20, 27.65, 27.96, 28.14, 28.65, 31.26, 31.41, 32.67, 34.29, 34.81, 37.13, 38.06, 38.66, 39.83, 41.17, 49.02, 49.45, 50.47, 52.08, 54.56, 56.10, 70.18, 70.99, 81.52, 85.47, 86.57, 175.68.
(20S,24S)-Epoxy-3β-O-(L-lysyl)-dammarane-12,25β-diol (26). White solid (yield 47%); m.p. 191–194 °C, HR-MS (HESI) m/z calcd. for C36H64N2O5 [M + H]+: 605.48880, found: 605.48895; 1H-NMR (CDCl3, 400 MHz) δ (ppm) 0.82 (3H, s, -CH3), 0.84 (3H, s, -CH3), 0.88 (6H, s, -CH3), 0.88 (6H, s, -CH3), 0.98 (3H, s, -CH3), 1.07 (3H, s, -CH3), 1.20 (6H, s, -CH3), 1.22 (3H, s, -CH3), 1.24 (3H, s, -CH3), 2.67 (2H, t, J = 6.6 Hz, NCH2-), 3.39 (1H, dd, J = 7.3 Hz, J = 5.2 Hz, -NCH-), 3.50 (1H, dt, J = 10.2 Hz, J = 5.1 Hz -OCH-), 3.84 (1H, dd, J = 10.8 Hz, J = 5.4 Hz -OCH-), 4.49 (1H, t, J = 12.0 Hz, -OCH-)13C-NMR (CDCl3, 100 MHz) δ (ppm) 15.56, 16.41, 16.68, 17.91, 22.10, 23.69, 23.80, 24.18, 26.91, 27.97, 28.16, 28.96, 29.78, 31.75, 31.94, 32.00, 32.33, 34.77, 34.84, 37.14, 38.05, 38.59, 39.41, 39.84, 48.87, 49.00, 50.19, 52.24, 53.61, 56.01, 70.26, 70.56, 82.75, 87.20, 87.53, 172.95.