Relationship between the Polymeric Ionization Degree and Powder and Surface Properties in Materials Derived from Poly(maleic anhydride-alt-octadecene)
Abstract
:1. Introduction
2. Results and Discussion
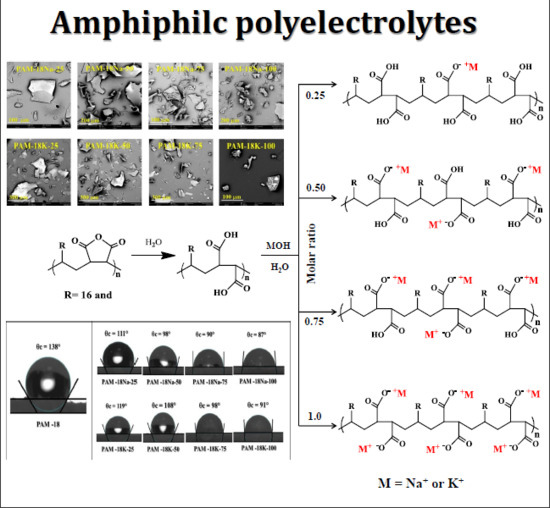
2.1. Obtention of Polymeric Materials Derived from PAM-18
2.2. Determination of Ionization Degree and Zeta-Potential
2.3. Structural Characterization of Polymer Materials
2.3.1. Fourier Transform Infrared Spectroscopy (FT-IR) Characterization
2.3.2. X-ray Diffraction (XRD)
2.3.3. Thermogravimetric Analysis (TGA)
2.3.4. Differential Scanning Calorimetry (DSC) Analysis
2.4. Characterization of Powder Polymeric Materials
2.4.1. Powder Morphology and Shape
2.4.2. Flowability Assays
2.4.3. Humidity Loss and Gain Study
2.5. Surface Polymer Characterization
3. Materials and Methods
3.1. Materials
3.2. Obtention of Polymeric Materials Derived from PAM-18
3.3. Determination of Ionization Degree and Zeta-Potential
3.4. Structural Characterizations of Polymeric Materials
3.4.1. FT-IR Characterization
3.4.2. X-ray Diffraction (XRD)
3.4.3. Thermal Analysis
3.5. Characterization of Powder Polymeric Materials
3.5.1. External Morphology Description
3.5.2. Particle Characterization
3.5.3. Humidity Loss and Gain Studies
3.6. Polymeric Surface Characterization
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Trivedi, B.C.; Culbertson, B.M. Maleic Anhydride; Springer: New York, NY, USA, 1982; Available online: http://link.springer.com/book/10.1007%2F978-1-4757-0940-7 (accessed on 31 January 2017).
- Martinez, F.; Uribe, E.; Olea, A.F. Copolymerization of maleic anhydride with styrene and α-Olefins. Molecular and thermal characterization. J. Macromol. Sci. Part A 2005, 2005, 1063–1072. [Google Scholar] [CrossRef]
- Salamanca, C.; Contreras, M.; Gamboa, C. Partial molar volume of anionic polyelectrolytes in aqueous solution. J. Colloid Interface Sci. 2007, 309, 435–439. [Google Scholar] [CrossRef] [PubMed]
- Salamanca, C.; Quintero, A.; Pineda, D.; Andrade, A. Aggregates of alternate amphiphilc polyanion to carry zwitterionic drug in aqueous media. Int. J. Pharm. Sci. Res. 2015, 6, 2360–2366. [Google Scholar]
- Barraza, R.G.; Olea, A.F.; Valdebenito, C.E.; Dougnac, V.; Fuentes, I. Solubilization ofp-nitrophenol in aggregates formed by hydrophobicallymodified polyelectrolytes. J. Colloid Interface Sci. 2004, 275, 434–438. [Google Scholar] [CrossRef] [PubMed]
- Salamanca, C.; Barraza, R.G.; Acevedo, B.; Olea, A.F. Hydrophobically modified polyelectrolytes as potential drugs reservoirs of N-alkyl-nitroimidazoles. J. Chil. Chem. Soc. 2007, 52. [Google Scholar] [CrossRef]
- Bacu, E.; Chitanu, G.C.; Couture, A.; Grandclaudon, P.; Singurel, G.; Carpov, A. Potential drug delivery systems from maleic anhydride copolymers and phenothiazine derivatives. Eur. Polym. J. 2002, 38, 1509–1513. [Google Scholar] [CrossRef]
- He, J.; Chen, D.; Fan, X.-M.; Wang, L.-W.; Deng, J.-Y.; Yang, W.-T. Reactive poly(divinyl benzene-co-maleic anhydride) nanoparticles: Preparation and characterization. Chin. Chem. Lett. 2013, 24, 970–974. [Google Scholar] [CrossRef]
- Arenas, T.; Mora, C.; Salamanca, C.H.; Jaramillo, M.C. Actividad del (2E)-3-(2,3-dimetoxifenil)-1-(4-metilfenil) prop-2-en-1-ona en presencia del poli(ácido maleico-co-2-vinil-pirrolidona) sobre un aislamiento clínico de Staphylococcus aureus productor de β-lactamasas. Iatreia 2012, 25, 12–19. [Google Scholar]
- Nitanan, T.; Akkaramongkolporn, P.; Rojanarata, T.; Ngawhirunpat, T.; Opanasopit, P. Neomycin-loaded poly(styrene sulfonic acid-co-maleic acid) (PSSA-MA)/polyvinyl alcohol (PVA) ion exchange nanofibers for wound dressing materials. Int. J. Pharm. 2013, 448, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Salamanca, C.; Urbano, B.; Olea, A.F. Potential drug delivery system: Study of the association of a model nitroimidazole drug with aggregates of amphiphilic polymers on aqueous solution. Braz. J. Pharm. Sci. 2011, 47, 6. [Google Scholar] [CrossRef]
- Song, S.; Liu, L.; Zhang, J. Annealing improves tribological property of poly(octadecene-alt-maleic anhydride) self-assembled film. Appl. Surf. Sci. 2011, 257, 10254–10260. [Google Scholar] [CrossRef]
- Winter, C.S.; Tredgold, R.H.; Vickers, A.J.; Khoshdel, E.; Hodge, P. Langmuir-Blodgett films from preformed polymers: Derivatives of octadec-1-ene-maleic anhydride copolymers. Thin Solid Films 1985, 134, 49–55. [Google Scholar] [CrossRef]
- El-Ghazawy, R.A.; Atta, A.M.; Kabel, K.I. Modified maleic anhydride-co-octadecene copolymers as flow improver for waxy Egyptian crude oil. J. Pet. Sci. Eng. 2014, 122, 411–419. [Google Scholar] [CrossRef]
- Di Corato, R.; Quarta, A.; Piacenza, P.; Ragusa, A.; Figuerola, A.; Buonsanti, R.; Cingolani, R.; Manna, L.; Pellegrino, T. Water solubilization of hydrophobic nanocrystals by means of poly(maleic anhydride-alt-1-octadecene). J. Mater. Chem. 2008, 18, 1991–1996. [Google Scholar] [CrossRef]
- Olea, A.F.; Gamboa, C. Synergism in mixtures of cationic surfactant and anionic copolymers. J. Colloid Interface Sci. 2003, 257, 321–326. [Google Scholar] [CrossRef]
- Olea, A.F.; Barraza, R.; Fuentes, I.; Acevedo, B. Solubilization of Phenols by Intramolecular Micelles Formed by Copolymers of Maleic Acid and Olefins. Macromolecules 2002, 35, 1049–1053. [Google Scholar] [CrossRef]
- Yarce, C.J.; Pineda, D.; Correa, C.E.; Salamanca, C.H. Relationship between surface properties and in Vitro drug release from a compressed matrix containing an amphiphilic polymer material. Pharmaceuticals 2016, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Yarce, C.J.; Echeverri, J.D.; Palacio, M.A.; Rivera, C.A.; Salamanca, C.H. Relationship between surface properties and in vitro drug release from compressed matrix containing polymeric materials with different hydrophobicity degrees. Pharmaceuticals 2017, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Barraza, R.G.; Olea, A.F.; Martinez, F.; Ruiz-Tagle, I. Adsorption of hydrophobically modified polyelectrolytes at the n-octane/water interface. J. Colloid Interface Sci. 2003, 261, 559–564. [Google Scholar] [CrossRef]
- Wu, H.L.; Wang, C.H.; Ma, C.C.M.; Chiu, Y.C.; Chiang, M.T.; Chiang, C.L. Preparations and properties of maleic acid and maleic anhydride functionalized multiwall carbon nanotube/poly(urea urethane) nanocomposites. Compos. Sci. Technol. 2007, 67, 1854–1860. [Google Scholar] [CrossRef]
- Elizondo, E.; Córdoba, A.; Sala, S.; Ventosa, N.; Veciana, J. Preparation of biodegradable poly (methyl vinyl ether-co-maleic anhydride) nanostructured microparticles by precipitation with a compressed antisolvent. J. Supercrit. Fluids 2010, 53, 108–114. [Google Scholar] [CrossRef]
- Zuo, Y.; Gu, J.; Yang, L.; Qiao, Z.; Tan, H.; Zhang, Y. Synthesis and characterization of maleic anhydride esterified corn starch by the dry method. Int. J. Biol. Macromol. 2013, 62, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Ngai, K.L.; Floudas, G.; Plazek, D.J.; Rizos, A.K. Amorphous Polymers. Encycl. Polym. Sci. Technol. 2002, 5, 63–111. [Google Scholar] [CrossRef]
- Schönhals, A.; Kremer, F. Amorphous Polymers. Polym. Sci. A Compr. Ref. 2012, 1, 201–226. [Google Scholar]
- Zhao, J.; Fan, Q. Amorphous phase in atactic polystyrene. Polym. Bull. 2001, 47, 91–97. [Google Scholar] [CrossRef]
- Yeh, G.S.Y. Morphology of amorphous polymers and effects of thermal and mechanical treatments on the morphology. Pure Appl. Chem. 1972, 65–89. [Google Scholar] [CrossRef]
- Gad, S.C. United States Pharmacopoeia (USP). In Encyclopedia of Toxicology, 3rd ed.; Wexler, P., Ed.; Academic Press: Oxford, UK, 2014; pp. 881–882. ISBN 978-0-12-386455-0. [Google Scholar]
- Grey, R.O.; Beddow, J.K. On the Hausner Ratio and its relationship to some properties of metal powders. Powder Technol. 1969, 2, 323–326. [Google Scholar] [CrossRef]
- Saw, H.Y.; Davies, C.E.; Paterson, A.H.J.; Jones, J.R. Correlation between powder flow properties measured by shear testing and Hausner ratio. Procedia Eng. 2015, 102, 218–225. [Google Scholar] [CrossRef]
- Leuenberger, H. The compressibility and compactibility of powder systems. Int. J. Pharm. 1982, 12, 41–55. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the author. |








| Polymeric System | Molar Ratio Polymer: Base | Polymer Ionization Degree (%) | pH ± SD | Zeta-Potential ± SD | |||
|---|---|---|---|---|---|---|---|
| PAM-18Na | PAM-18K | PAM-18Na | PAM-18K | PAM-18Na | PAM-18K | ||
| PAM-18M-100 | 1:1 | 95 | 99 | 11.2 ± 0.0 | 11.1 ± 0.0 | −59 ± 0.9 | −58.5 ± 0.1 |
| PAM-18M-75 | 1:0.75 | 63 | 52 | 10.6 ± 0.1 | 10.5 ± 0.0 | −49.3 ± 1.3 | −54.7 ± 3.3 |
| PAM-18M-50 | 1:0.5 | 39 | 35 | 8.6 ± 0.1 | 8.3 ± 0.0 | −37.7 ± 1.0 | −44.1 ± 1.6 |
| PAM-18M-25 | 1:0.25 | 22 | 20 | 6.7 ± 0.1 | 6.5 ± 0.1 | −37.9 ± 0.3 | −39.1 ± 2.1 |
| Polymer Material | Repose Angle (°) ± SD | Carr Index ± SD | Hausner Index ± SD |
|---|---|---|---|
| PAM-18 | 28 ± 0.5 | 20.7 ± 1.3 | 1.3 ± 0 |
| PAM-18Na-25 | 37.4 ± 1.3 | 22.6 ± 0 | 1.3 ± 0 |
| PAM-18Na-50 | 35.2 ± 0.6 | 18 ± 0 | 1.2 ± 0 |
| PAM-18Na-75 | 33.3 ± 1.1 | 17.8 ± 0 | 1.2 ± 0 |
| PAM-18Na-100 | 30.5 ± 1.6 | 16.9 ± 1 | 1.2 ± 0 |
| PAM-18K-25 | 45.1 ± 1 | 20 ± 0 | 1.3 ± 0 |
| PAM-18K-50 | 43 ± 1.4 | 16.1 ± 0 | 1.2 ± 0 |
| PAM-18K-75 | 39 ± 0.7 | 15.4 ± 0 | 1.2 ± 0 |
| PAM-18K-100 | 38.1 ± 1 | 11.6 ± 1.4 | 1.2 ± 0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salamanca, C.H.; Yarce, C.J.; Zapata, C.A.; Giraldo, J.A. Relationship between the Polymeric Ionization Degree and Powder and Surface Properties in Materials Derived from Poly(maleic anhydride-alt-octadecene). Molecules 2018, 23, 320. https://doi.org/10.3390/molecules23020320
Salamanca CH, Yarce CJ, Zapata CA, Giraldo JA. Relationship between the Polymeric Ionization Degree and Powder and Surface Properties in Materials Derived from Poly(maleic anhydride-alt-octadecene). Molecules. 2018; 23(2):320. https://doi.org/10.3390/molecules23020320
Chicago/Turabian StyleSalamanca, Constain H., Cristhian J. Yarce, Camilo A. Zapata, and Jonnathan A. Giraldo. 2018. "Relationship between the Polymeric Ionization Degree and Powder and Surface Properties in Materials Derived from Poly(maleic anhydride-alt-octadecene)" Molecules 23, no. 2: 320. https://doi.org/10.3390/molecules23020320
APA StyleSalamanca, C. H., Yarce, C. J., Zapata, C. A., & Giraldo, J. A. (2018). Relationship between the Polymeric Ionization Degree and Powder and Surface Properties in Materials Derived from Poly(maleic anhydride-alt-octadecene). Molecules, 23(2), 320. https://doi.org/10.3390/molecules23020320