Persicaline, A New Antioxidant Sulphur-Containing Imidazoline Alkaloid from Salvadora persica Roots
Abstract
:1. Introduction
2. Results
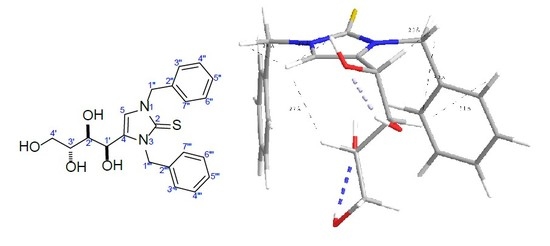
2.1. Isolation, and Sructure Elucidation
2.2. Antioxidant Assays
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Plant Material
4.3. Extraction, Fraction, and Isolation
4.4. Antioxidant Assay
4.4.1. DPPH Scavenging Radical Assay
4.4.2. Superoxide Anion Scavenging Radical Assay
4.4.3. Nitric Oxide Scavenging Radical Assay
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mabberley, D.J. Mabberley’s Plant Book, a Portable Dictionary of Plants, Their Classifications and Uses, 3rd ed.; Cambridge University Press: Cambridge, UK, 2008; p. 845. ISBN 10: 0521820715. [Google Scholar]
- Mathur, S.; Shekhawat, G.S.; Batra, A. Micropropagation of Salvadora persica via cotyledonary nodes. Ind. J. Biotech. 2002, 1, 197–200. [Google Scholar]
- Kamil, M.; Jayaraj, A.F.; Ahmed, F.; Gunasekhar, C.; Samuel, S.; Habibullah, M.; Chan, K.J. Pharmacognostic and phytochemical studies on Salvadorapersica. Pharm. Pharmacol. 1999, 227, 51–58. [Google Scholar]
- Akhtar, M.; Ajmal, M.J. Significance of chewing-sticks (miswaks) in oral hygiene from a pharmacological view-point. Pak. Med. Assoc. 1981, 31, 89–95. [Google Scholar]
- Al Lafi, T.; Ababneh, H. The effect of the extract of the miswak (chewing sticks) used in Jordan and the Middle East on oral bacteria. Int. Dent J. 1995, 45, 218–222. [Google Scholar] [PubMed]
- Farooqi, M.; Srivastava, J.J. The toothbrush tree (Salvadora persica). Crude. Drug. Res. 1968, 8, 1297–1299. [Google Scholar] [CrossRef]
- Galletti, G.C.; Chiavari, G.; Kahie, Y.D. pyrolysis/gas chromatography/ion-trap mass spectrometry of the ‘tooth brush’tree (Salvadora persica L.). Rap. Commun. Mass Spect. 1993, 7, 651–656. [Google Scholar] [CrossRef]
- Ohtani, K.; Kasai, R.; Yamasaki, K.; Tanaka, O.; Kamel, M.S.; Assaf, M.H.; El-Shanawani, M.A.; Ali, A.A. Lignan glycosides from stems of Salvadora persica. Phytochemistry 1992, 31, 2469–2471. [Google Scholar] [CrossRef]
- Aumeeruddya, M.Z.; Zenginb, G.; Mahomoodally, M.F. A review of the traditional and modern uses of Salvadora persica L. (Miswak): Toothbrush tree of Prophet Muhammad. J. Ethnopharm. 2017, 213, 409–444. [Google Scholar] [CrossRef] [PubMed]
- Farag, M.A.; Fahmy, S.; Choucry, M.A.; Wahdan, M.O.; Elsebai, M.F. Metabolites profiling reveals for antimicrobial compositional differences and action mechanism in the toothbrushing stick miswak Salvadora persica. J. Pharm. Biomed. Anal. 2017, 133, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Yusuf, M.; Al Robaian, M.; Ali, M. Isolation and characterization of four novel β-Sitosteryl esters from Salvadora persica Linn. Nat. Prod. Res. 2017, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wasimuzzama, K.; Mujib, A.; Tausif, S.; Rashmi, T.; Katekar, S.; Rukhsana, A. Phytochemical and pharmacological profile of miswak (Salvadora persica Linn Salvadoraceae): An overview. Pharma. Online 2010, 2, 534–548. [Google Scholar]
- Abdel-Kader, M.S.; Muharram, M.M.; Foudah, A.I.; Alqarni, M.H.; Salkini, M.A. Antimicrobial isothiocyanate derivatives from Salvadora persica root “Siwak” extract. Indo. Am. J. Pharm. Sci. 2017, 4, 1224–1228. [Google Scholar]
- Khalil, A.T. Benzylamides from Salvadora persica. Arch. Pharm. Res. 2006, 29, 952–956. [Google Scholar] [CrossRef] [PubMed]
- Shachi, S.; Swapnil, V.S.K. Antibacterial properties of Alkaloid rich fractions obtained from various parts of Prosopis juliflora. Int. J. Pharm. Sci. Res. 2011, 2, 114–120. [Google Scholar]
- Trzhtsinskaya, B.V.; Abramova, N.D. Imidazole-2-thione: Synthesis, structure, properties. Sulf. Rep. 1991, 10, 389–421. [Google Scholar] [CrossRef]
- Rao, C.R.; Venkataraghavan, R. The C=S stretching frequency and the “-N-C=S bands” in the infrared. Spectrochem. Acta 1962, 18, 541–547. [Google Scholar] [CrossRef]
- Akira, M.; Yumi, K.; Suratwadee, J.; Koichi, K.; Hajime, O. Niaziminin, a thiocarbamate from the Leaves of Moringa oleifera, holds a strict structural requirement for inhibition of tumor-promoter-induced Epstein-Barr virus activation. Planta Med. 1998, 64, 319–923. [Google Scholar]
- Ilias, M.; Jianping, Z.O.; Chuck, D.D.; Ikhlas, A.K. Constituents of Lepidium meyenii ‘maca’. Phytochemistry 2002, 59, 105–110. [Google Scholar]
- Parthasarathy, S.; Bin Azizi, J.; Ramanathan, S.; Ismail, S.; Sasidharan, S.; Said, M.I.; Mansor, S.M. Evaluation of antioxidant and antibacterial activities of aqueous, methanolic and alkaloid extracts from Mitragyna Speciosa (Rubiaceae family) leaves. Molecules 2009, 14, 3964–3974. [Google Scholar] [CrossRef] [PubMed]
- Shekhar, T.C.; Anju, G. Antioxidant activity by DPPH radical scavenging method of Ageratum conyzoides Linn. Leaves. Am. J. Ethnomed. 2014, 1, 244–249. [Google Scholar]
- Ibrahim, M.M.; AL Sahli, A.A.; Alaraidh, I.A.; Al-Homaidan, A.A.; Mostafa, E.M.; EL-Gaaly, G.A. Assessment of antioxidant activities in roots of Miswak (Salvadora persica) plants grown at two different locations in Saudi Arabia. Saud. J. Biol. Sci. 2015, 22, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Fontana, M.; Mosca, L.; Rosei, M.A. Interaction of enkephailns with oxyradicals. Biochem. Pharma. 2001, 61, 1253–1257. [Google Scholar] [CrossRef]
- Nagmoti, D.M.; Khatri, D.K.; Juvekar, P.R.; Juvekar, A.R. Antioxidant activity and free radical scavenging potential of Pithecellobium dulce Benth seed extracts. Free Rad. Antiox. 2011, 2, 37–43. [Google Scholar] [CrossRef]
- Servillo, L.; Balestrieri, M.L.; Casale, R.; Onofrio, D.N.; Giovane, A.; Cautela, D.; Castaldo, D. Uncommon redox behaviour enlightens the cellular antioxidant properties of ergothioneine. Free Rad. Biol. Med. 2015, 79, 228–236. [Google Scholar] [CrossRef] [PubMed]
- Gua, Q.L.; Lin, S.; Wang, Y.N.; Zhu, C.G.; Xu, C.B.; Shi, J.G. Gastrolatathioneine, an unusual ergothioneine derivative from an aqueous extract of “Tian ma”: A natural product co-produced by plant and symbiotic fungus. Chin. Chem. Lett. 2016, 27, 1577–1581. [Google Scholar] [CrossRef]
- Arimurea, G.I.; Maffei, M. Plant Specialized Metabolism: Genomics, Biochemistry and Biological Functions, 1st ed.; CRC press: Broken Sound Parkway, NW, USA, 2017; p. 1957. ISBN 9781498726283. [Google Scholar]
- Tao, X.L.; Lei, M.; Wang, Y.G. Unexpected microwave reaction of 1,3-disubstituted imidazolium salts: A novel synthesis of 1,3-disubstituted imidazole-2-thiones. Syn. Commun. 2007, 37, 399–408. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1–6 are available from the authors. |




| Position | Compound 1 | |
|---|---|---|
| δH | δC | |
| 1 | ||
| 2 | 162.7 C | |
| 3 | ||
| 4 | 131.7 C | |
| 5 | 7.08, s | 115.8 CH |
| 1′ | 4.62, m | 62.9 CH |
| 2′ | 3.38, m | 71.9 CH |
| 3′ | 3.40, m | 70.7 CH |
| 4′ | a 3.53, m | 63.3 CH2 |
| b 3.37, m | ||
| 1′′ | a 5.32, (d, 15.9) | 49.9 CH2 |
| b 5.23, (d, 15.9) | ||
| 2′′ | 137.1 C | |
| 3′′ | 7.36, (d, 7.5) | 127.9 CH |
| 4′′ | 7.31, (d, 7.5) | 128.4 CH |
| 5′′ | 7.31, m | 127.7 CH |
| 6′′ | 7.31, (d, 7.5) | 128.4 CH |
| 7′′ | 7.36, (d, 7.5) | 127.9 CH |
| 1′′′ | 5.42, s | 47.6 CH2 |
| 2′′′ | 137.1 C | |
| 3′′′ | 7.22, (d, 7.5) | 126.8 CH |
| 4′′′ | 7.37, (d, 7.5) | 128.6 CH |
| 5′′′ | 7.25, m | 127.2 CH |
| 6′′′ | 7.37, (d, 7.5) | 128.6 CH |
| 7′′′ | 7.22, (d, 7.5) | 126.8 CH |
| OH-1′ | 5.11, (d, 8.1) | |
| OH-2′ | 4.68, (d, 8.1) | |
| OH-3′ | 4.55, (d, 8.1) | |
| OH-4′ | 4.35, (t, 5.6) | |
| Sample (µg/mL) | % Inhibition ± SD | |||
|---|---|---|---|---|
| Total Alcohol Extract | Non-Basic Fraction | Compound 1 | Ascorbic Acid | |
| 6.25 | 1 ± 0.2 | 10.2 ± 0.95 | 8.8 ± 0.82 | 40.32 ± 0.05 |
| 12.5 | 2.4 ± 0.03 | 27.91 ± 8.83 | 13.68 ± 1.91 | 94.22 ± 0.04 |
| 25 | 4 ± 0.01 | 31.12 ± 1.7 | 25.07 ± 0.89 | 96.56 ± 0.04 |
| 50 | 7.5 ± 0.01 | 46.1 ± 16.69 | 61.30 ± 13.48 | 96.86 ± 0.02 |
| 100 | 15.5 ± 0.14 | 71.94 ± 0.34 | 60.65 ± 1.46 | 96.76 ± 0.02 |
| Sample (µg/mL) | % Inhibition ± SD | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Alcohol Extract | Pet. Ether Fraction | Non Basic Fraction | Alkaloid-Rich Fraction | Isolated Compounds | Ascorbic Acid | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | ||||||
| 6.25 | 9.56 ± 3.09 | 6.49 ± 3.02 | 29.61 ± 7.77 | 12.93 ± 6.73 | 21.74 ± 14.25 | 5.99 ± 2.86 | - | 13.13 ± 3.75 | 0.46 ± 0.80 | 3.50 ± 2.35 | 57.10 ± 6.51 |
| 12.5 | 33.00 ± 11.85 | 16.16 ± 3.74 | 47.35 ± 7.31 | 27.63 ± 10.78 | 32.89 ± 11.41 | 10.02 ± 2.14 | - | 34.55 ± 13.91 | 4.00 ± 3.19 | 9.03 ± 1.56 | 66.16 ± 6.64 |
| 25 | 56.50 ± 6.17 | 26.20 ± 1.65 | 62.20 ± 3.67 | 40.83 ± 10.00 | 51.41 ± 13.73 | 19.19 ± 3.37 | - | 44.00 ± 7.01 | 4.50 ± 3.05 | 17.03 ± 4.36 | 75.36 ± 6.98 |
| 50 | 75.83 ± 9.04 | 46.80 ± 8.02 | 72.13 ± 2.62 | 55.90 ± 0.63 | 58.80 ± 15.63 | 32.80 ± 4.24 | - | 57.17 ± 7.49 | 6.50 ± 4.65 | 22.13 ± 3.69 | 84.36 ± 5.49 |
| 100 | 86.96 ± 4.99 | 61.47 ± 10.60 | 82.33 ± 1.33 | 66.16 ± 5.31 | 72.30 ± 5.32 | 42.47 ± 6.32 | - | 71.26 ± 6.42 | 7.76 ± 4.31 | 26.19 ± 2.70 | 88.40 ± 5.04 |
| Sample (µg/mL) | % Inhibition ± SD | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Alcohol Extract | Pet. Ether Fraction | Non Basic Fraction | Alkaloid-Rich Fraction | Isolated Compounds | Ascorbic Acid | ||||||
| 1 | 2 | 3 | 4 | 5 | 6 | ||||||
| 6.25 | 31.43 ± 13.05 | 5.16 ± 2.48 | 7.40 ± 4.56 | 10.56 ± 5.34 | 17.86 ± 15.85 | 10.40 ± 6.32 | 3.68 ± 2.57 | 6.66 ± 2.10 | 0.72 ± 1.25 | 7.40 ± 4.56 | 23.86 ± 8.91 |
| 12.5 | 51.43 ± 3.14 | 10.43 ± 3.25 | 20.06 ± 8.58 | 23.09 ± 3.04 | 28.10 ± 22.28 | 14.16 ± 4.35 | 10.30 ± 2.62 | 11.26 ± 0.28 | 1.46 ± 1.26 | 12.63 ± 5.43 | 41.43 ± 7.77 |
| 25 | 63.69 ± 10.70 | 19.36 ± 8.72 | 36.71 ± 16.53 | 33.50 ± 8.52 | 42.40 ± 23.65 | 25.61 ± 5.18 | 10.28 ± 1.22 | 19.20 ± 6.40 | 3.66 ± 1.25 | 19.43 ± 6.21 | 48.16 ± 7.16 |
| 50 | 71.60 ± 10.48 | 21.63 ± 10.28 | 50.50 ± 10.21 | 50.03 ± 4.00 | 59.16 ± 14.84 | 30.03 ± 3.35 | 11.73 ± 3.21 | 21.86 ± 5.35 | 5.13 ± 1.20 | 28.46 ± 12.0 | 66.03 ± 8.24 |
| 100 | 76.83 ± 5.01 | 27.00 ± 7.85 | 57.00 ± 10.21 | 56.54 ± 6.03 | 70.36 ± 14.73 | 33.06 ± 1.86 | 14.68 ± 5.02 | 25.61 ± 5.18 | 6.61 ± 0.08 | 38.30 ± 5.63 | 77.36 ± 4.22 |
| Compound/Fraction | IC50 µg/mL (µM) in DPPH Assay | IC50 µg/mL (µM) in Superoxide Anion Assay | IC50 µg/mL (µM) in Nitric Oxide Assay |
|---|---|---|---|
| Total alcohol extract | 327.7 | 20 | 12 |
| Pet. Ether fraction | 399.1 | 60 | 201.4 |
| Non-basic fraction | 60.0 | 15.6 | 48 |
| Alkaloid-rich fraction | 126.3 | 36 | 50 |
| 1 | 38.5 (0.1) | 32.7(0.08) | 34.5 (0.09) |
| 2 | 1551.4 (7.9) | 46.5 (0.24) | 161.0 (0.82) |
| 3 | 1659.4 (4.8) | Inactive | 502.3 (1.45) |
| 4 | 666.2 (4.5) | 36 (0.24) | 223.9 (1.5) |
| 5 | Inactive | 757.5 (3.36) | 809.6 (3.59) |
| 6 | 1180 (7.9) | 196.5 (1.31) | 130.2 (0.87) |
| Ascorbic Acid | 7.0 (0.04) | 1.5 (0.01) | 28 (0.16) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Farag, M.; Abdel-Mageed, W.M.; Basudan, O.; El-Gamal, A. Persicaline, A New Antioxidant Sulphur-Containing Imidazoline Alkaloid from Salvadora persica Roots. Molecules 2018, 23, 483. https://doi.org/10.3390/molecules23020483
Farag M, Abdel-Mageed WM, Basudan O, El-Gamal A. Persicaline, A New Antioxidant Sulphur-Containing Imidazoline Alkaloid from Salvadora persica Roots. Molecules. 2018; 23(2):483. https://doi.org/10.3390/molecules23020483
Chicago/Turabian StyleFarag, Mohamed, Wael M. Abdel-Mageed, Omer Basudan, and Ali El-Gamal. 2018. "Persicaline, A New Antioxidant Sulphur-Containing Imidazoline Alkaloid from Salvadora persica Roots" Molecules 23, no. 2: 483. https://doi.org/10.3390/molecules23020483
APA StyleFarag, M., Abdel-Mageed, W. M., Basudan, O., & El-Gamal, A. (2018). Persicaline, A New Antioxidant Sulphur-Containing Imidazoline Alkaloid from Salvadora persica Roots. Molecules, 23(2), 483. https://doi.org/10.3390/molecules23020483