Graphene Oxide as a Nanocarrier for a Theranostics Delivery System of Protocatechuic Acid and Gadolinium/Gold Nanoparticles
Abstract
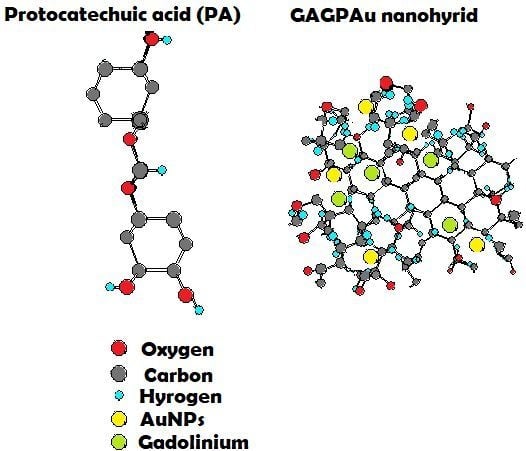
:1. Introduction
2. Results and Discussion
2.1. Protocatechuic Acid Release Pattern from GAGPA Nanocomposite
Protocatechuic Acid Release Kinetics from GAGPA Nanocomposite
2.2. Powder X-ray Diffraction Studies
2.3. Raman Spectroscopy Studies
2.4. Thermal Studies
2.5. Fourier Transformed Infrared Spectroscopy Analysis
2.6. Transmission Electron Microscopy Studies
2.7. Cytotoxicity Studies
2.8. Magnetic Resonance Imaging Studies
3. Materials and Methods
3.1. Materials
3.2. Characterization
3.3. Graphene Oxide (GO) Synthesis
3.4. Synthesis of Graphene Oxide—Gadolinium and Protocatechuic Acid Nanocomposite
3.5. Synthesis of Gold Nanoparticles on the GAGPA Nanocomposite Surface
3.6. Drug Loading and Release from GAGPA Nanocomposite
3.7. Cytotoxicity Study
3.7.1. Cell Culture
3.7.2. Cytotoxicity Evaluation
3.8. Magnetic Resonance Imaging Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Usman, M.S.; Ibrahim, N.A.; Shameli, K.; Zainuddin, N.; Yunus, W.M.Z.W. Copper nanoparticles mediated by chitosan: synthesis and characterization via chemical methods. Molecules 2012, 17, 14928–14936. [Google Scholar] [CrossRef] [PubMed]
- Hancock, Y. The 2010 Nobel Prize in physics—Ground-breaking experiments on graphene. J. Phys. D 2011, 44, 473001. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [PubMed]
- Goenka, S.; Sant, V.; Sant, S. Graphene-based nanomaterials for drug delivery and tissue engineering. J. Control. Release 2014, 173, 75–88. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-S.; Lu, Y.-J.; Chen, J.-P. Magnetic graphene oxide as a carrier for targeted delivery of chemotherapy drugs in cancer therapy. J. Magn. Magn. Mater. 2017, 427, 34–40. [Google Scholar] [CrossRef]
- Kim, J.; Cote, L.J.; Kim, F.; Yuan, W.; Shull, K.R.; Huang, J. Graphene oxide sheets at interfaces. J. Am. Chem. Soc. 2010, 132, 8180–8186. [Google Scholar] [CrossRef] [PubMed]
- Usman, M.S.; Hussein, M.Z.; Fakurazi, S.; Ahmad Saad, F.F. Gadolinium-based layered double hydroxide and graphene oxide nano-carriers for magnetic resonance imaging and drug delivery. Chem. Cent. J. 2017, 11, 47. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Kattel, K.; Park, J.Y.; Chang, Y.; Kim, T.J.; Lee, G.H. Paramagnetic nanoparticle T1 and T2 MRI contrast agents. Phys. Chem. Chem. Phys. 2012, 14, 12687–12700. [Google Scholar] [CrossRef] [PubMed]
- Sani Usman, M.; Hussein, M.Z.; Fakurazi, S.; Masarudin, M.J.; Ahmad Saad, F.F. Gadolinium-Doped Gallic Acid-Zinc/Aluminium-Layered Double Hydroxide/Gold Theranostic Nanoparticles for a Bimodal Magnetic Resonance Imaging and Drug Delivery System. Nanomaterials 2017, 7, 244. [Google Scholar] [CrossRef] [PubMed]
- Carr, D.; Brown, J.; Bydder, G.; Steiner, R.; Weinmann, H.; Speck, U.; Hall, A.; Young, I. Gadolinium-DTPA as a contrast agent in MRI: Initial clinical experience in 20 patients. Am. J. Roentgenol. 1984, 143, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Nguyen Ngoc, L.; Le Van, V.; Chu Dinh, K.; Sai Cong, D.; Cao Thi, N.; Pham Thi, H.; Nguyen Duy, T.; Luu Manh, Q. Synthesis and optical properties of colloidal gold nanoparticles. J. Phys. Conf. Ser. 2009, 187, 012026. [Google Scholar]
- Balcioglu, M.; Rana, M.; Yigit, M.V. Doxorubicin loading on graphene oxide, iron oxide and gold nanoparticle hybrid. J. Mater. Chem. B 2013, 1, 6187–6193. [Google Scholar] [CrossRef]
- Sun, X.; Liu, Z.; Welsher, K.; Robinson, J.T.; Goodwin, A.; Zaric, S.; Dai, H. Nano-graphene oxide for cellular imaging and drug delivery. Nano Res. 2008, 3, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.-C.; Lin, C.-C.; Wu, H.-C.; Tsao, S.-M.; Hsu, C.-K. Apoptotic effects of protocatechuic acid in human breast, lung, liver, cervix, and prostate cancer cells: Potential mechanisms of action. J. Agric. Food Chem. 2009, 57, 6468–6473. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.-Y.; Yin, M.-C. Antibacterial effects of roselle calyx extracts and protocatechuic acid in ground beef and apple juice. Foodborne Pathog. Dis. 2009, 6, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Jaijoy, K.; Soonthornchareonnon, N.; Panthong, A.; Sireeratawong, S. Anti-inflammatory and analgesic activities of the water extract from the fruit of Phyllanthus emblica Linn. Int. J. Appl. Res. Nat. Prod. 2010, 3, 28–35. [Google Scholar]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano. 2010, 4, 4806–4814. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Bao, Y.-M.; Jiang, B.; An, L.-J. Protective effect of protocatechuic acid from Alpinia oxyphylla on hydrogen peroxide-induced oxidative PC12 cell death. Eur. J. Pharmacol. 2006, 538, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Sahu, A.; Choi, W.I.; Lee, J.H.; Tae, G. Graphene oxide mediated delivery of methylene blue for combined photodynamic and photothermal therapy. Biomaterials 2013, 34, 6239–6248. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, P. Chemotherapy* A2—HIMMELWEIT, F. In The Collected Papers of Paul Ehrlich; Pergamon: Oxford, UK, 1960; pp. 505–518. [Google Scholar]
- Zhang, B.; Yang, X.; Wang, Y.; Zhai, G. Heparin modified graphene oxide for pH-sensitive sustained release of doxorubicin hydrochloride. Mater. Sci. Eng. C 2017, 75, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Barahuie, F.; Hussein, M.Z.; Arulselvan, P.; Fakurazi, S.; Zainal, Z. Drug delivery system for an anticancer agent, chlorogenate-Zn/Al-layered double hydroxide nanohybrid synthesised using direct co-precipitation and ion exchange methods. J. Solid State Chem. 2014, 217, 31–41. [Google Scholar] [CrossRef]
- Dorniani, D.; Saifullah, B.; Barahuie, F.; Arulselvan, P.; Hussein, M.Z.B.; Fakurazi, S.; Twyman, L.J. Graphene Oxide-Gallic Acid Nanodelivery System for Cancer Therapy. Nanoscale Res. Lett. 2016, 11, 491. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.A.; Lee, W.C.; Shi, H.; Wong, E.Y.; Sadovoy, A.; Gorelik, S.; Hobley, J.; Lim, C.T.; Loh, K.P. Highly wrinkled cross-linked graphene oxide membranes for biological and charge-storage applications. Small 2012, 8, 423–431. [Google Scholar] [CrossRef] [PubMed]
- Rana, V.K.; Choi, M.C.; Kong, J.Y.; Kim, G.Y.; Kim, M.J.; Kim, S.H.; Mishra, S.; Singh, R.P.; Ha, C.S. Synthesis and drug-delivery behavior of chitosan-functionalized graphene oxide hybrid nanosheets. Macromol. Mater. Eng. 2011, 296, 131–140. [Google Scholar] [CrossRef]
- Barahuie, F.; Hussein, M.; Fakurazi, S.; Zainal, Z. Development of Drug Delivery Systems Based on Layered Hydroxides for Nanomedicine. Int. J. Mol. Sci. 2014, 15, 7750–7786. [Google Scholar] [CrossRef] [PubMed]
- Barahuie, F.; Dorniani, D.; Saifullah, B.; Gothai, S.; Hussein, M.Z.; Pandurangan, A.K.; Arulselvan, P.; Norhaizan, M.E. sustained release of anticancer agent phytic acid from its chitosan-coated magnetic nanoparticles for drug-delivery system. Int. J. Nanomed. 2017, 12, 2361. [Google Scholar] [CrossRef] [PubMed]
- Nasir, S.; Hussein, M.Z.; Yusof, N.A.; Zainal, Z. Oil Palm Waste-Based Precursors as a Renewable and Economical Carbon Sources for the Preparation of Reduced Graphene Oxide from Graphene Oxide. Nanomaterials 2017, 7, 182. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Sun, X.; Liu, Y.; Wang, W.; Qiu, H.; Gao, J. One pot preparation of reduced graphene oxide (RGO) or Au (Ag) nanoparticle-RGO hybrids using chitosan as a reducing and stabilizing agent and their use in methanol electrooxidation. Carbon 2012, 50, 2513–2523. [Google Scholar] [CrossRef]
- Verma, S.; Mungse, H.P.; Kumar, N.; Choudhary, S.; Jain, S.L.; Sain, B.; Khatri, O.P. Graphene oxide: an efficient and reusable carbocatalyst for aza-Michael addition of amines to activated alkenes. Chem. Commun. 2011, 47, 12673–12675. [Google Scholar] [CrossRef] [PubMed]
- Kuila, T.; Bose, S.; Khanra, P.; Kim, N.H.; Rhee, K.Y.; Lee, J.H. Characterization and properties of in situ emulsion polymerized poly (methyl methacrylate)/graphene nanocomposites. Compos. Part A Appl. Sci. Manuf. 2011, 42, 1856–1861. [Google Scholar] [CrossRef]
- Bi, X.; Zhang, H.; Dou, L. Layered double hydroxide-based nano-carriers for drug delivery. Pharmaceutics 2014, 6, 298–332. [Google Scholar] [CrossRef] [PubMed]
- Barahuie, F.; Hussein, M.Z.; Gani, S.A.; Fakurazi, S.; Zainal, Z. Synthesis of protocatechuic acid–zinc/aluminium-layered double hydroxide nanocomposite as an anticancer nanodelivery system. J. Solid State Chem. 2015, 221, 21–31. [Google Scholar] [CrossRef]
- Eisenberg, D.; Eisenberg, D.S.; Kauzmann, W. The Structure and Properties of Water; Oxford University Press on Demand: Oxford, UK, 2005. [Google Scholar]
- Sun, G.; Zhou, L.; Liu, Y.; Zhao, Z. Biocompatible Gd III-functionalized fluorescent gold nanoclusters for optical and magnetic resonance imaging. New J. Chem. 2013, 37, 1028–1035. [Google Scholar] [CrossRef]
- Shen, A.J.; Li, D.L.; Cai, X.J.; Dong, C.Y.; Dong, H.Q.; Wen, H.Y.; Dai, G.H.; Wang, P.J.; Li, Y.Y. Multifunctional nanocomposite based on graphene oxide for in vitro hepatocarcinoma diagnosis and treatment. J. Biomed. Mater. Res. A 2012, 100, 2499–2506. [Google Scholar] [CrossRef] [PubMed]
- Barahuie, F.; Saifullah, B.; Dorniani, D.; Fakurazi, S.; Karthivashan, G.; Hussein, M.Z.; Elfghi, F.M. Graphene oxide as a nanocarrier for controlled release and targeted delivery of an anticancer active agent, chlorogenic acid. Mater. Sci. Eng. C 2017, 74, 177–185. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Li, Y.; Jia, X.-R.; Du, J.; Ying, X.; Lu, W.-L.; Lou, J.-N.; Wei, Y. PEGylated Poly (amidoamine) dendrimer-based dual-targeting carrier for treating brain tumors. Biomaterials 2011, 32, 478–487. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Zong, C.; Shen, H.; Liu, M.; Chen, B.; Ren, B.; Zhang, Z. Mechanism of Cellular Uptake of Graphene Oxide Studied by Surface-Enhanced Raman Spectroscopy. Small 2012, 8, 2577–2584. [Google Scholar] [CrossRef] [PubMed]
- Manus, L.M.; Mastarone, D.J.; Waters, E.A.; Zhang, X.Q.; Schultz-Sikma, E.A.; MacRenaris, K.W.; Ho, D.; Meade, T.J. Gd (III)-nanodiamond conjugates for MRI contrast enhancement. Nano Lett. 2009, 10, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Pylypchuk, I.V.; Kołodyńska, D.; Kozioł, M.; Gorbyk, P. Gd-DTPA Adsorption on Chitosan/Magnetite Nanocomposites. Nanoscale Res. Lett. 2016, 11, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, H.; Wang, J.; Yang, Y.; Wu, D.; Zhang, Y.; Zhang, Y.; Zhou, Z.; Yang, S. Graphene oxide-BaGdF 5 nanocomposites for multi-modal imaging and photothermal therapy. Biomaterials 2015, 42, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, T.; Xiao, Y.; Yu, D.; Zhang, N. Hyaluronic Acid-Chitosan Nanoparticles to Deliver Gd-DTPA for MR Cancer Imaging. Nanomaterials 2015, 5, 1379–1396. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.E.; Zeng, L.; Pan, Y.; Luo, S.; Ren, W.; Gong, A.; Ma, X.; Liang, H.; Lu, G.; Wu, A. Inorganic photosensitizer coupled Gd-based upconversion luminescent nanocomposites for in vivo magnetic resonance imaging and near-infrared-responsive photodynamic therapy in cancers. Biomaterials 2015, 44, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Luo, K.; Tian, J.; Liu, G.; Sun, J.; Xia, C.; Tang, H.; Lin, L.; Miao, T.; Zhao, X.; Gao, F. Self-assembly of SiO2/Gd-DTPA-polyethylenimine nanocomposites as magnetic resonance imaging probes. J. Nanosci. Nanotechnol. 2010, 10, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Hedlund, A.; Ahrén, M.; Gustafsson, H.; Abrikossova, N.; Warntjes, M.; Jönsson, J.I.; Uvdal, K.; Engström, M. Gd2O3 nanoparticles in hematopoietic cells for MRI contrast enhancement. Int. J. Nanomed. 2011, 6, 3233. [Google Scholar]
- Fortin, M.A.; Petoral, R.M.; Söderlind, F.; Klasson, A.; Engström, M.; Veres, T.; Käll, P.O.; Uvdal, K. Polyethylene glycol-covered ultra-small Gd2O3 nanoparticles for positive contrast at 1.5 T magnetic resonance clinical scanning. Nanotechnology 2007, 18, 395501. [Google Scholar] [CrossRef]
- Bellin, M.F. Gadolinium-Based Contrast Agents. In Contrast Media: Safety Issues and ESUR Guidelines; Thomsen, H.S., Webb, J.A.W., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 199–204. [Google Scholar]
- Atabaev, T.; Lee, J.; Shin, Y.; Han, D.-W.; Choo, K.; Jeon, U.; Hwang, J.; Yeom, J.; Kim, H.-K.; Hwang, Y.-H. Eu, Gd-Codoped Yttria Nanoprobes for Optical and T1-Weighted Magnetic Resonance Imaging. Nanomaterials 2017, 7, 35. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Peng, E.; Zheng, B.; Li, S.F.Y.; Xue, J.M. Synthesis of Water-Dispersible Gd2O3/GO Nanocomposites with Enhanced MRI T 1 Relaxivity. J. Phys. Chem. C 2015, 119, 23735–23742. [Google Scholar] [CrossRef]
- Le, W.; Cui, S.; Chen, X.; Zhu, H.; Chen, B.; Cui, Z. Facile Synthesis of Gd-Functionalized Gold Nanoclusters as Potential MRI/CT Contrast Agents. Nanomaterials 2016, 6, 65. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xing, H.; Zhang, S.; Ren, Q.; Pan, L.; Zhang, K.; Bu, W.; Zheng, X.; Zhou, L.; Peng, W. A Gd-doped Mg-Al-LDH/Au nanocomposite for CT/MR bimodal imagings and simultaneous drug delivery. Biomaterials 2013, 34, 3390–3401. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.P.; Kurniawan, N.D.; Bartlett, P.F.; Lu, G.Q. Enhancement of relaxivity rates of Gd–DTPA complexes by intercalation into layered double hydroxide nanoparticles. Chem. Eur. J. 2007, 13, 2824–2830. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are available from the authors. |







| Sample pH | Correlation Coefficients (R2) | Percentage Saturation (%) | Rate Constant (k) | t1/2 (min) | ||
|---|---|---|---|---|---|---|
| Pseudo-First Order | Pseudo-Second Order | Parabolic Diffusion | ||||
| 7.4 | 0.863 | 0.992 | 0.936 | 50 | 90 | |
| 4.8 | 0.563 | 0.985 | 0.932 | 80 | 170 | |
| Sample | Trange (°C) | Tmax (°C) | Delta m (%) |
|---|---|---|---|
| GO (A) | 71–255 | 197 | 57 |
| Protocatechuic acid (B) | 122–308 | 262 | 88 |
| GAGPA (C) | 38–888 | 200 | 68 |
| GAGPAu (D) | 73–747 | 537 | 58 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usman, M.S.; Hussein, M.Z.; Kura, A.U.; Fakurazi, S.; Masarudin, M.J.; Ahmad Saad, F.F. Graphene Oxide as a Nanocarrier for a Theranostics Delivery System of Protocatechuic Acid and Gadolinium/Gold Nanoparticles. Molecules 2018, 23, 500. https://doi.org/10.3390/molecules23020500
Usman MS, Hussein MZ, Kura AU, Fakurazi S, Masarudin MJ, Ahmad Saad FF. Graphene Oxide as a Nanocarrier for a Theranostics Delivery System of Protocatechuic Acid and Gadolinium/Gold Nanoparticles. Molecules. 2018; 23(2):500. https://doi.org/10.3390/molecules23020500
Chicago/Turabian StyleUsman, Muhammad Sani, Mohd Zobir Hussein, Aminu Umar Kura, Sharida Fakurazi, Mas Jaffri Masarudin, and Fathinul Fikri Ahmad Saad. 2018. "Graphene Oxide as a Nanocarrier for a Theranostics Delivery System of Protocatechuic Acid and Gadolinium/Gold Nanoparticles" Molecules 23, no. 2: 500. https://doi.org/10.3390/molecules23020500
APA StyleUsman, M. S., Hussein, M. Z., Kura, A. U., Fakurazi, S., Masarudin, M. J., & Ahmad Saad, F. F. (2018). Graphene Oxide as a Nanocarrier for a Theranostics Delivery System of Protocatechuic Acid and Gadolinium/Gold Nanoparticles. Molecules, 23(2), 500. https://doi.org/10.3390/molecules23020500