Mechanistic Insight into the Degradation of Nitrosamines via Aqueous-Phase UV Photolysis or a UV-Based Advanced Oxidation Process: Quantum Mechanical Calculations
Abstract
:1. Introduction
2. Results and Discussion
2.1. HO•-Induced Degradation
2.1.1. N-Nitrosodimethylamine (NDMA) Degradation Pathways Induced by HO•
2.1.2. N-Nitrosomethylethylamine (NMEA) Degradation Pathways Induced by HO•
2.1.3. N-Nitrosomethylbutylamine (NMBA) Degradation Pathways Induced by HO•
2.2. UV-Induced Degradation
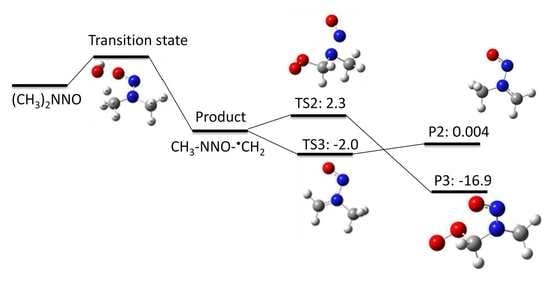
NDMA Degradation Pathways Induced by UV Photolysis
2.3. Environmental Implication and Future Study
3. Materials and Methods
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
List of Symbols and Abbreviations
| AOPs | advanced oxidation processes |
| CH2=N(+)HCH3 | N-methylidenemethylamine |
| CH3NHCH3 | methyl diamine |
| DFT | density functional theory |
| theoretically calculated aqueous phase free energy of activation | |
| theoretically calculated aqueous phase free energy of reaction | |
| G4 | Gaussian-4 theory |
| HCHO | formaldehyde |
| HCOOH | formic acid |
| HNO2 | nitrous acid |
| HO• | hydroxyl radicals |
| HOMO | highest occupied molecular orbital |
| NF | nanofiltration |
| NDEA | N-nitrosodiethylamine |
| NDMA | N-nitrosodimethylamine |
| NMEA | N-nitrosomethylethylamine |
| NO• | nitric oxide |
| NO3− | nitrate ion |
| NO2− | nitrite ion |
| ONOO− | peroxynitrite |
| •O2− | superoxide anion radical |
| QM | quantum mechanical |
| RO | reverse osmosis |
| SMD | universal solvation model |
| TD-DFT | time-dependent density functional theory |
| TS | transition state |
| UV | ultraviolet |
References
- US EPA. Technical Fact Sheet N-Nitroso-Dimethylamine (NDMA); EPA 505-F-17-005, November 2017; EPA: Washington, DC, USA, 2017.
- Fine, D.H.; Rounbehler, D.P.; Rounbehler, A.; Silvergleid, A.; Sawicki, E.; Krost, K.; Demarrais, G.A. Determination of dimethylnitrosamine in air, water, and soil by thermal-energy analysis- measurements in baltimore, md. Environ. Sci. Technol. 1977, 11, 581–584. [Google Scholar] [CrossRef]
- California Environmental Protection Agency, State Water Resrouces Control Board. NDMA and Other Nitrosamines-Drinking Water Issues. Available online: https://www.waterboards.ca.gov/drinking_water/certlic/drinkingwater/NDMA.shtml (accessed on 6 February 2018).
- Glaze, W.H.; Kang, J.W. Advanced oxidation processes- test of a kinetic-model for the oxidation of organic-compounds with ozone and hydrogen-peroxide in a semibatch reactor. Ind. Eng. Chem. Res. 1989, 28, 1580–1587. [Google Scholar] [CrossRef]
- Glaze, W.H.; Kang, J.W.; Chapin, D.H. The chemistry of water-treatment processes involving ozone, hydrogen-peroxide and ultraviolet-radiation. Ozone Sci. Eng. 1987, 9, 335–352. [Google Scholar] [CrossRef]
- Tchobanoglous, G. Framework for Direct Potable Reuse; WateReuse Research Foundation: Alexandria, VA, USA, 2015. [Google Scholar]
- Mitch, W.A.; Sharp, J.O.; Trussell, R.R.; Valentine, R.L.; Alvarez-Cohen, L.; Sedlak, D.L. N-nitrosodimethylamine (NDMA) as a drinking water contaminant: A review. Environ. Eng. Sci. 2003, 20, 389–404. [Google Scholar] [CrossRef]
- Landsman, N.A.; Swancutt, K.L.; Bradford, C.N.; Cox, C.R.; Kiddle, J.J.; Mezyk, S.P. Free radical chemistry of advanced oxidation process removal of nitrosamines in water. Environ. Sci. Technol. 2007, 41, 5818–5823. [Google Scholar] [CrossRef] [PubMed]
- Mezyk, S.P.; Cooper, W.J.; Madden, K.P.; Bartels, D.M. Free radical destruction of N-nitrosodimethylamine in water. Environ. Sci. Technol. 2004, 38, 3161–3167. [Google Scholar] [CrossRef] [PubMed]
- Chow, Y.L. Nitrosamine photochemistry-reactions of aminium radicals. Acc. Chem. Res. 1973, 6, 354–360. [Google Scholar] [CrossRef]
- Chow, Y.L.; Tam, J.N.S.; Lau, M.P.; Perry, R.A. Photoreactions of nitroso-compounds in solution. XX. Photoreduction, photoelimination, and photoaddition of nitrosamines. Can. J. Chem. 1972, 50, 1044–1050. [Google Scholar] [CrossRef]
- Daiber, D.; Preussmann, R. Quantitative colorimetrische bestimmung organischer n-nitroso-verbindungen durch photochemische spaltung der nitrosaminbindung (quantitative colorimetric determination of organic n-nitroso compounds by photochemical splitting of the nitroso amine bond). Fresenius Z. Anal. Chem. 1964, 206, 344–352. [Google Scholar] [CrossRef]
- Grilli, S.; Tosi, M.R.; Prodi, G. Degradation of dimethylnitrosoamine catalyzed by physical and chemical agents. Gann 1975, 66, 481–488. [Google Scholar] [PubMed]
- Stefan, M.I.; Bolton, J.R. Uv direct photolysis of N-nitrosodimethylamine (NDMA): Kinetic and product study. Helv. Chim. Acta 2002, 85, 1416–1426. [Google Scholar] [CrossRef]
- Lee, C.; Choi, W.; Kim, Y.G.; Yoon, J. Uv photolytic mechanism of N-nitrosodimethylamine in water: Dual pathways to methylamine versus dimethylamine. Environ. Sci. Technol. 2005, 39, 2101–2106. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Choi, W.; Yoon, J. Uv photolytic mechanism of N-nitrosodimethylamine in water: Roles of dissolved oxygen and solution ph. Environ. Sci. Technol. 2005, 39, 9702–9709. [Google Scholar] [CrossRef] [PubMed]
- Kwon, B.G.; Kim, J.-O.; Namkung, K.C. The formation of reactive species having hydroxyl radical-like reactivity from UV photolysis of N-nitrosodimethylamine (NDMA): Kinetics and mechanism. Sci. Total Environ. 2012, 437, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Kwon, B.G.; Kim, J.-O.; Namkung, K.C. Formation of reactive species enhanced by H2O2 addition in the photodecomposition of N-nitrosodimethylamine (NDMA). Environ. Eng. Res. 2013, 18, 29–35. [Google Scholar] [CrossRef]
- Lee, C.; Yoon, J.; Von Gunten, U. Oxidative degradation of N-nitrosodimethylamine by conventional ozonation and the advanced oxidation process ozone/hydrogen peroxide. Water Res. 2007, 41, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Li, Y.M.; Song, Y. Reinvestigation on the ozonation of N-nitrosodimethylamine: Influencing factors and degradation mechanism. Water Res. 2013, 47, 4993–5002. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.Y.; Noerpel, M.; Luk, H.L.; Wei, Z.S.; Spinney, R. Thermodynamic and kinetic study of ibuprofen with hydroxyl radical: A density functional theory approach. Int. J. Quantum Chem. 2014, 114, 74–83. [Google Scholar] [CrossRef]
- An, T.C.; Gao, Y.P.; Li, G.Y.; Kamat, P.V.; Peller, J.; Joyce, M.V. Kinetics and mechanisms of •OH mediated degradation of dimethyl phthalate in aqueous solution: Experimental and theoretical studies. Environ. Sci. Technol. 2014, 48, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Q.; Zhou, X.Z.; Han, W.Q.; Li, J.S.; Sun, X.Y.; Shen, J.Y.; Wang, L.J. Theoretical and experimental insights into the •OH-medited mineralization mechanism of flutriafol. Electrochim. Acta 2017, 235, 223–232. [Google Scholar] [CrossRef]
- Trogolo, D.; Mishra, B.K.; Heeb, M.B.; von Gunten, U.; Arey, J.S. Molecular mechanism of ndma formation from N,N-dimethylsulfamide during ozonation: Quantum chemical insights into a bromide-catalyzed pathway. Environ. Sci. Technol. 2015, 49, 4163–4175. [Google Scholar] [CrossRef] [PubMed]
- Minakata, D.; Crittenden, J. Linear free energy relationships between aqueous phase hydroxyl radical reaction rate constants and free energy of activation. Environ. Sci. Technol. 2011, 45, 3479–3486. [Google Scholar] [CrossRef] [PubMed]
- Minakata, D.; Song, W.H.; Crittenden, J. Reactivity of aqueous phase hydroxyl radical with halogenated carboxylate anions: Experimental and theoretical studies. Environ. Sci. Technol. 2011, 45, 6057–6065. [Google Scholar] [CrossRef] [PubMed]
- Minakata, D.; Song, W.H.; Mezyk, S.P.; Cooper, W.J. Experimental and theoretical studies on aqueous-phase reactivity of hydroxyl radicals with multiple carboxylated and hydroxylated benzene compounds. Phys. Chem. Chem. Phys. 2015, 17, 11796–11812. [Google Scholar] [CrossRef] [PubMed]
- Minakata, D.; Mezyk, S.P.; Jones, J.W.; Daws, B.R.; Crittenden, J.C. Development of linear free energy relationships for aqueous phase radical-involved chemical reactions. Environ. Sci. Technol. 2014, 48, 13925–13932. [Google Scholar] [CrossRef] [PubMed]
- Buxton, G.V.; Stuart, C.R. Radiation chemistry of aqueous solutions of hydrazine at elevated temperatures. J. Chem. Soc. Faraday Trans. 1997, 93, 1535–1538. [Google Scholar] [CrossRef]
- McMurry, J. Nucleophilic addition of H2O: Hydration. In Organic Chemistry; Cengage Learning: Boston, MA, USA, 2015. [Google Scholar]
- Bothe, E.; Schultefrohlinde, D. Reaction of dihydroxymethyl radical with molecular-oxygen in aqueous-solution. Z. Naturforsch. B 1980, 35, 1035–1039. [Google Scholar] [CrossRef]
- Von Sonntag, C.; Schuchmann, H.P. The elucidation of peroxyl radical reactions in aqueous-solution with the help of radiation-chemical methods. Angew. Chem. Int. Ed. 1991, 30, 1229–1253. [Google Scholar] [CrossRef]
- Padmaja, S.; Huie, R.E. The reaction of nitric-oxide with organic peroxyl radicals. Biochem. Biophys. Res. Commun. 1993, 195, 539–544. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.; Czapski, G. The reaction of NO with O2 and HO: A pulse radiolysis study. Free Radic. Biol. Med. 1995, 19, 505–510. [Google Scholar] [CrossRef]
- Anbar, M.; Taube, H. Interaction of nitrous acid with hydrogen peroxide and with water. J. Am. Chem. Soc. 1954, 76, 6243–6247. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.02; Gaussian Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Curtiss, L.A.; Redfern, P.C.; Raghavachari, K. Gaussian-4 theory. J. Chem. Phys. 2007, 126, 84108. [Google Scholar] [CrossRef] [PubMed]
- Marenich, A.V.; Cramer, C.J.; Truhlar, D.G. Universal solvation model based on solute electron density and a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. J. Phys. Chem. B 2009, 113, 6378–6396. [Google Scholar] [CrossRef] [PubMed]
- Adamo, C.; Jacquemin, D. The calculations of excited-state properties with Time-Dependent Density Functional Theory. Chem. Soc. Rev. 2013, 42, 845–856. [Google Scholar] [CrossRef] [PubMed]
- Laurent, A.D.; Adamo, C.; Jacquemin, D. Dye chemistry with time-dependent density functional theory. Phys. Chem. Chem. Phys. 2014, 16, 1434–14356. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |















© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Minakata, D.; Coscarelli, E. Mechanistic Insight into the Degradation of Nitrosamines via Aqueous-Phase UV Photolysis or a UV-Based Advanced Oxidation Process: Quantum Mechanical Calculations. Molecules 2018, 23, 539. https://doi.org/10.3390/molecules23030539
Minakata D, Coscarelli E. Mechanistic Insight into the Degradation of Nitrosamines via Aqueous-Phase UV Photolysis or a UV-Based Advanced Oxidation Process: Quantum Mechanical Calculations. Molecules. 2018; 23(3):539. https://doi.org/10.3390/molecules23030539
Chicago/Turabian StyleMinakata, Daisuke, and Erica Coscarelli. 2018. "Mechanistic Insight into the Degradation of Nitrosamines via Aqueous-Phase UV Photolysis or a UV-Based Advanced Oxidation Process: Quantum Mechanical Calculations" Molecules 23, no. 3: 539. https://doi.org/10.3390/molecules23030539
APA StyleMinakata, D., & Coscarelli, E. (2018). Mechanistic Insight into the Degradation of Nitrosamines via Aqueous-Phase UV Photolysis or a UV-Based Advanced Oxidation Process: Quantum Mechanical Calculations. Molecules, 23(3), 539. https://doi.org/10.3390/molecules23030539