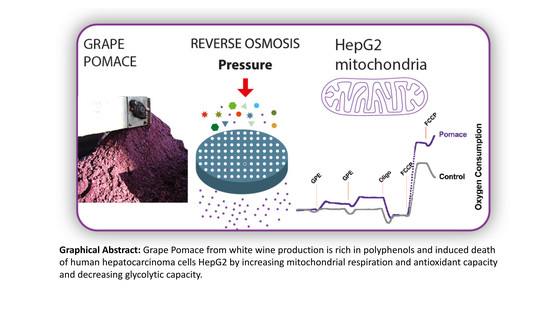
Anthocyanin-Rich Grape Pomace Extract (Vitis vinifera L.) from Wine Industry Affects Mitochondrial Bioenergetics and Glucose Metabolism in Human Hepatocarcinoma HepG2 Cells
Abstract
:1. Introduction
2. Results
2.1. Pinot Noir Grape Pomace Extract (GPE) Is Highly Concentrated in Phenolic Compounds and Anthocyanins
2.2. GPE Induced Alterations in HepG2 Morphology
2.3. Pinot Noir Grape Pomace Extract (GPE) Was Cytotoxic to HepG2 Cells
2.4. GPE Increased Mitochondrial Respiration and Antioxidant Capacity in HepG2 Cells
2.5. Grape Pomace Extract Decreased Glycolytic Flux and Affected Intracellular Metabolites in HepG2 Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Grape Pomace
4.3. Chemical Assays
4.3.1. ABTS Radical TEAC Assay
4.3.2. ORAC Assay
4.3.3. FRAP Assay
4.3.4. Total Phenolic Content
4.3.5. Total Flavonoid Content
4.3.6. Total and Monomeric Anthocyanin Content
4.3.7. Anthocyanins Profile in GPE by HPLC-PDA
4.4. Cellular Assays
4.4.1. Cell Culture
4.4.2. Cytotoxicity Assays
4.4.3. Cell Death by Flow Cytometry
4.4.4. Cellular Antioxidant Activity
4.4.5. Oxygen Consumption Analysis and Calculation of Respiratory Parameters
4.4.6. Glucose Uptake and Lactate Efflux to the Culture Medium
4.4.7. Nuclear Magnetic Resonance (NMR) for Analysis of Intracellular Metabolites
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rodríguez-Enríquez, S.; Marín-Hernández, A.; Gallardo-Pérez, J.C.; Carreño-Fuentes, L.; Moreno-Sánchez, R. Targeting of cancer energy metabolism. Mol. Nutr. Food Res. 2009, 53, 29–48. [Google Scholar] [CrossRef] [PubMed]
- González-Vallinas, M.; González-Castejón, M.; Rodríguez-Casado, A.; Molina, A.R. Dietary phytochemicals in cancer prevention and therapy: A complementary approach with promising perspectives. Nutr. Rev. 2013, 71, 585–599. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, M.R.; Nabavi, S.F.; Manayi, A.; Daglia, M.; Hajheydari, Z.; Nabavi, S.M. Resveratrol and the mitochondria: From triggering the intrinsic apoptotic pathway to inducing mitochondrial biogenesis, a mechanistic view. Biochim. Biophys. Acta 2016, 1860, 727–745. [Google Scholar] [CrossRef] [PubMed]
- Gorlach, S.; Fichna, J.; Lewandowska, U. Polyphenols as mitochondria-targeted anticancer drugs. Cancer Lett. 2015, 366, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Acuña, C.; Ferreira, J.; Speisky, H. Polyphenols and mitochondria: An update on their increasingly emerging ROS-scavenging independent actions. Arch. Biochem. Biophys. 2014, 559, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.K.; George, J.; Ahmad, N. Resveratrol-based combinatorial strategies for cancer management. Ann. N. Y. Acad. Sci. 2013, 1290, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Vinod, B.S.; Maliekal, T.T.; Anto, R.J. Phytochemicals As Chemosensitizers: From Molecular Mechanism to Clinical Significance. Antioxid. Redox Signal. 2013, 18, 1307–1348. [Google Scholar] [CrossRef] [PubMed]
- Instituto Brasileiro do Vinho—IBRAVIN. Produção de Vinhos e Derivados. 2017. Available online: http://www.ibravin.org.br/admin/arquivos/estatisticas/1502979131.pdf (accessed on 24 October 2017).
- Bustamante, M.A.; Moral, R.; Paredes, C.; Pérez-Espinosa, A.; Moreno-Caselles, J.; Pérez-Murcia, M.D. Agrochemical characterisation of the solid by-products and residues from the winery and distillery industry. Waste Manag. 2008, 28, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Antoniolli, A.; Fontana, A.R.; Piccoli, P.; Bottini, R. Characterization of polyphenols and evaluation of antioxidant capacity in grape pomace of the cv. Malbec. Food Chem. 2015, 178, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Inada, K.O.P.; Oliveira, A.A.; Revorêdo, T.B.; Martins, A.B.N.; Lacerda, E.C.Q.; Freire, A.S.; Braz, B.F.; Santelli, R.E.; Torres, A.G.; Perrone, D.; et al. Screening of the chemical composition and occurring antioxidants in jabuticaba (Myrciaria jaboticaba) and jussara (Euterpe edulis) fruits and their fractions. J. Funct. Foods 2015, 17, 422–433. [Google Scholar] [CrossRef]
- Kammerer, D.; Claus, A.; Carle, R.; Schieber, A. Polyphenol screening of pomace from red and white grape varieties (Vitis vinifera L.) by HPLC-DAD-MS/MS. J. Agric. Food Chem. 2004, 52, 4360–4367. [Google Scholar] [CrossRef] [PubMed]
- Makris, D.P.; Kefalas, P. Characterization of Polyphenolic Phytochemicals in Red Grape Pomace. Int. J. Waste Resour. 2013, 3, 126. [Google Scholar] [CrossRef]
- Rockenbach, I.I.; Gonzaga, L.V.; Rizelio, V.M.; Gonçalves, A.E.d.S.S.; Genovese, M.I.; Fett, R. Phenolic compounds and antioxidant activity of seed and skin extracts of red grape (Vitis vinifera and Vitis labrusca) pomace from Brazilian winemaking. Food Res. Int. 2011, 44, 897–901. [Google Scholar] [CrossRef]
- Rockenbach, I.I.; Rodrigues, E.; Gonzaga, L.V.; Caliari, V.; Genovese, M.I.; de Souza Schmidt Gonçalves, A.E.; Fett, R. Phenolic compounds content and antioxidant activity in pomace from selected red grapes (Vitis vinifera L. and Vitis labrusca L.) widely produced in Brazil. Food Chem. 2011, 127, 174–179. [Google Scholar] [CrossRef]
- Ruberto, G.; Renda, A.; Daquino, C.; Amico, V.; Spatafora, C.; Tringali, C.; de Tommasi, N. Polyphenol constituents and antioxidant activity of grape pomace extracts from five Sicilian red grape cultivars. Food Chem. 2007, 100, 203–210. [Google Scholar] [CrossRef]
- Sri Harsha, P.S.C.; Gardana, C.; Simonetti, P.; Spigno, G.; Lavelli, V. Characterization of phenolics, in vitro reducing capacity and anti-glycation activity of red grape skins recovered from winemaking by-products. Bioresour. Technol. 2013, 140, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.P.G. [Recovery of Bioactive Compounds from Winemaking by-Products] Recuperacao de Compostos Bioativos a Partir de Residuos da Industria Vitivinicola. Ph.D. Thesis, Federal University of Rio de Janeiro, Rio de Janeiro, Brazil, 2013. [Google Scholar]
- Gao, H.; Wang, H.; Peng, J. Hispidulin Induces Apoptosis through Mitochondrial Dysfunction and Inhibition of P13k/Akt Signalling Pathway in HepG2 Cancer Cells. Cell Biochem. Biophys. 2014, 69, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Choi, Y.; Ham, H.; Jeong, H.S.; Lee, J. Protective effects of oligomeric and polymeric procyanidin fractions from defatted grape seeds on tert-butyl hydroperoxide-induced oxidative damage in HepG2 cells. Food Chem. 2013, 137, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Ramos, A.A.; Lima, C.F.; Pereira, M.L.; Fernandes-Ferreira, M.; Pereira-Wilson, C. Antigenotoxic effects of quercetin, rutin and ursolic acid on HepG2 cells: Evaluation by the comet assay. Toxicol. Lett. 2008, 177, 66–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jara-Palacios, M.J.; Hernanz, D.; Cifuentes-Gomez, T.; Escudero-Gilete, M.L.; Heredia, F.J.; Spencer, J.P.E. Assessment of white grape pomace from winemaking as source of bioactive compounds, and its antiproliferative activity. Food Chem. 2015, 183, 78–82. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, M.F.; Amoêdo, N.D.; Rumjanek, F.D. Energy and redox homeostasis in tumor cells. Int. J. Cell Biol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Trumbeckaité, S.; Burdulis, D.; Raudoné, L.; Liobikas, J.; Toleikis, A.; Janulis, V. Direct Effects of Vaccinium myrtillus L. Fruit Extracts on Rat Heart Mitochondrial Functions. Phytother. Res. 2013, 27, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Skemiene, K.; Liobikas, J.; Borutaite, V. Anthocyanins as substrates for mitochondrial complex I—Protective effect against heart ischemic injury. FEBS J. 2015, 282, 963–971. [Google Scholar] [CrossRef] [PubMed]
- Amoêdo, N.D.; Rodrigues, M.F.; Pezzuto, P.; Galina, A.; da Costa, R.M.; de Almeida, F.C.L.; El-Bacha, T.; Rumjanek, F.D. Energy metabolism in H460 lung cancer cells: Effects of histone deacetylase inhibitors. PLoS ONE 2011, 6, e22264. [Google Scholar] [CrossRef] [PubMed]
- Pereira da Silva, A.P.; El-Bacha, T.; Kyaw, N.; dos Santos, R.S.; Da-Silva, W.S.; Almeida, F.C.L.; Da Poian, A.T.; Galina, A. Inhibition of energy-producing pathways of HepG2 cells by 3-bromopyruvate. Biochem. J. 2009, 417, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.S.; Giampieri, F.; Janjusevic, M.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Mazzoni, L.; Greco, S.; Giannubilo, S.R.; Ciavattini, A.; Mezzetti, B.; et al. An anthocyanin rich strawberry extract induces apoptosis and ROS while decreases glycolysis and fibrosis in human uterine leiomyoma cells. Oncotarget 2017, 8, 23575–23587. [Google Scholar] [CrossRef] [PubMed]
- Cruz, A.P.G.; Silva e Sousa, C.G.; Torres, A.G.; Freitas, S.P.; Cabral, L.M.C. Recuperação De Compostos Bioativos a Partir Do Bagaço De Uva. Rev. Bras. Frutic. 2013, 35, 1147–1157. [Google Scholar] [CrossRef]
- Zulueta, A.; Esteve, M.J.; Frígola, A. ORAC and TEAC assays comparison to measure the antioxidant capacity of food products. Food Chem. 2009, 114, 310–316. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [CrossRef] [PubMed]
- Georgé, S.; Brat, P.; Alter, P.; Amiot, M.J. Rapid determination of polyphenols and vitamin C in plant-derived products. J. Agric. Food Chem. 2005, 53, 1370–1373. [Google Scholar] [CrossRef] [PubMed]
- Kao, T.H.; Chen, B.H. Functional components in soybean cake and their effects on antioxidant activity. J. Agric. Food Chem. 2006, 54, 7544–7555. [Google Scholar] [CrossRef] [PubMed]
- Giusti, M.M.; Wrosltad, R.E. Characterization and measurement of anthocyanins by UV-visible spectroscopy. In Current Protocols in Food Analytical Chemistry; Wrolstad, R.E., Ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2001. [Google Scholar] [CrossRef]
- Alcalde-Eon, C.; Escribano-Bailón, M.T.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. Changes in the detailed pigment composition of red wine during maturity and ageing: A comprehensive study. Anal. Chim. Acta 2006, 563, 238–254. [Google Scholar] [CrossRef]
- Saavedra, J.; Fuentealba, C.; Yáñez, M.; Bravo, L.; Quiroz, W.; Lukacsy, G.; Carot, J.M. Chemometric approaches for the zoning of Pinot Noir wines from the Casablanca valley, Chile. Food Chem. 2011, 127, 1842–1847. [Google Scholar] [CrossRef]
- Dimitrovska, M.; Bocevska, M.; Dimitrovski, D.; Murkovic, M. Anthocyanin composition of Vranec, Cabernet Sauvignon, Merlot and Pinot Noir grapes as indicator of their varietal differentiation. Eur. Food Res. Technol. 2011, 232, 591–600. [Google Scholar] [CrossRef]
- Wolfe, K.L.; Liu, R.H. Cellular Antioxidant Activity (CAA) Assay for Assesing Antioxidants, Foods, and Dietary Supplements. J. Agric. Food Chem. 2007, 55, 8896–8907. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Vazquez, E.J.; Moghaddas, S.; Hoppel, C.L.; Lesnefsky, E.J. Production of reactive oxygen species by mitochondria: Central role of complex III. J. Biol. Chem. 2003, 278, 36027–36031. [Google Scholar] [CrossRef] [PubMed]
- Gnaiger, E. Capacity of oxidative phosphorylation in human skeletal muscle. New perspectives of mitochondrial physiology. Int. J. Biochem. Cell Biol. 2009, 41, 1837–1845. [Google Scholar] [CrossRef] [PubMed]
- Brand, M.D.; Nicholls, D.G. Assessing mitochondrial dysfunction in cells. Biochem. J. 2011, 435, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Silva da Costa, L.; Pereira da Silva, A.P.; Da Poian, A.T.; El-Bacha, T. Mitochondrial bioenergetic alterations in mouse neuroblastoma cells infected with sindbis virus: Implications to viral replication and neuronal death. PLoS ONE 2012, 7, e33871. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S.; Tzur, D.; Knox, C.; Eisner, R.; Guo, A.C.; Young, N.; Cheng, D.; Jewell, K.; Arndt, D.; Sawhney, S.; et al. HMDB: The human metabolome database. Nucleic Acids Res. 2007, 35, D521–D526. [Google Scholar] [CrossRef] [PubMed]
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the grape pomace are available from the authors. |







| Phenolic Compounds (Mean ± SE; mg × 100 g−1 DM a) | Antioxidant CAPACITY (Mean ± SE; µmol × L−1 × g−1 DM) | |||||
|---|---|---|---|---|---|---|
| TPC b | Total Flavonoids c | Total Anthocyanins d | Monomeric Anthocyanins d | ABTS e | FRAP f | ORAC e |
| 34,061 ± 860.1 | 2146 ± 110.6 | 1582 ± 7.4 | 1441 ± 8.2 | 1014 ± 0.1 | 3220 ± 6.4 | 167.1 ± 1.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Sales, N.F.F.; Silva da Costa, L.; Carneiro, T.I.A.; Minuzzo, D.A.; Oliveira, F.L.; Cabral, L.M.C.; Torres, A.G.; El-Bacha, T. Anthocyanin-Rich Grape Pomace Extract (Vitis vinifera L.) from Wine Industry Affects Mitochondrial Bioenergetics and Glucose Metabolism in Human Hepatocarcinoma HepG2 Cells. Molecules 2018, 23, 611. https://doi.org/10.3390/molecules23030611
De Sales NFF, Silva da Costa L, Carneiro TIA, Minuzzo DA, Oliveira FL, Cabral LMC, Torres AG, El-Bacha T. Anthocyanin-Rich Grape Pomace Extract (Vitis vinifera L.) from Wine Industry Affects Mitochondrial Bioenergetics and Glucose Metabolism in Human Hepatocarcinoma HepG2 Cells. Molecules. 2018; 23(3):611. https://doi.org/10.3390/molecules23030611
Chicago/Turabian StyleDe Sales, Nathalia F. F., Leandro Silva da Costa, Talita I. A. Carneiro, Daniela A. Minuzzo, Felipe L. Oliveira, Lourdes M. C. Cabral, Alexandre G. Torres, and Tatiana El-Bacha. 2018. "Anthocyanin-Rich Grape Pomace Extract (Vitis vinifera L.) from Wine Industry Affects Mitochondrial Bioenergetics and Glucose Metabolism in Human Hepatocarcinoma HepG2 Cells" Molecules 23, no. 3: 611. https://doi.org/10.3390/molecules23030611
APA StyleDe Sales, N. F. F., Silva da Costa, L., Carneiro, T. I. A., Minuzzo, D. A., Oliveira, F. L., Cabral, L. M. C., Torres, A. G., & El-Bacha, T. (2018). Anthocyanin-Rich Grape Pomace Extract (Vitis vinifera L.) from Wine Industry Affects Mitochondrial Bioenergetics and Glucose Metabolism in Human Hepatocarcinoma HepG2 Cells. Molecules, 23(3), 611. https://doi.org/10.3390/molecules23030611