Identification of the NADPH Oxidase 4 Inhibiting Principle of Lycopus europaeus
Abstract
:1. Introduction
2. Results
2.1. Preparation of Extracts of L. europaeus and Investigation of Their NADPH Oxidase 4 (Nox4) Inhibitory Activities
2.2. Phytochemical Investigation of the Methanolic Extract of the Subaerial Parts of L. europaeus
2.3. Quantification of Rosmarinic Acid in L. europaeus Plant Material and Extracts
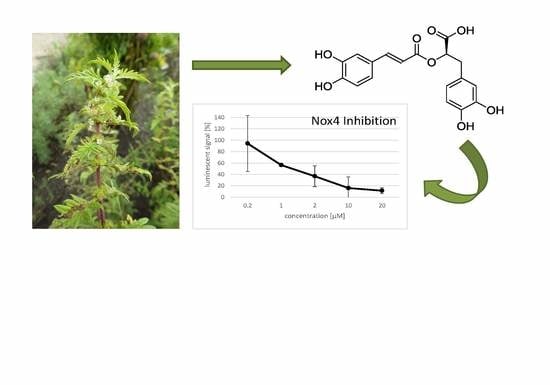
2.4. Effect of Rosmarinic Acid on Nox4
2.5. Effect of Rosmarinic Acid on Nox2 and Nox5 Activity
2.6. Effect of Rosmarinic Acid on Mitochondrial ROS Production
3. Discussion
4. Materials and Methods
4.1. Chemicals and Solvents
4.2. Plant Material
4.3. Extract Preparation for Isolation and Pharmacological Investigations
4.4. Isolation and Structure Elucidation
4.5. Quantification of Rosmarinic Acid in Aerial and Subaerial Parts of L. europaeus and Dry Extracts
4.6. Cell Viability Assays
4.6.1. MTT Assay
4.6.2. Hoechst 33342 Staining
4.7. Nox4 Assay
4.8. Nox2 Assay
4.9. Nox5 Assay
4.10. Mitochondrial ROS Assay
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kofler, P.A.; Pricher, H.; von Grafenstein, S.; Diener, T.; Hoell, M.; Liedl, K.R.; Siems, K.; Jansen-Duerr, P. Characterisation of Nox4 inhibitors from edible plants. Planta Med. 2013, 79, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Thallas-Bonke, V.; Jandeleit-Dahm, K.A.; Cooper, M.E. Nox-4 and progressive kidney disease. Curr. Opin. Nephrol. Hypertens. 2015, 24, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Nlandu-Khodo, S.; Dissard, R.; Hasler, U.; Schafer, M.; Pircher, H.; Jansen-Durr, P.; Krause, K.H.; Martin, P.Y.; de Seigneux, S. NADPH oxidase 4 deficiency increases tubular cell death during acute ischemic reperfusion injury. Sci. Rep. 2016, 6, 38598. [Google Scholar] [CrossRef] [PubMed]
- Hecker, L.; Logsdon, N.J.; Kurundkar, D.; Kurundkar, A.; Bernard, K.; Hock, T.; Meldrum, E.; Sanders, Y.Y.; Thannickal, V.J. Reversal of persistent fibrosis in aging by targeting Nox4-Nrf2 redox imbalance. Sci. Transl. Med. 2014, 6, 231ra47. [Google Scholar] [CrossRef] [PubMed]
- Jiang, F.; Liu, G.S.; Dusting, G.J.; Chan, E.C. NADPH oxidase-dependent redox signaling in TGF-beta-mediated fibrotic responses. Redox Biol. 2014, 2, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Sampson, N.; Koziel, R.; Zenzmaier, C.; Bubendorf, L.; Plas, E.; Jansen-Durr, P.; Berger, P. ROS signaling by Nox4 drives fibroblast-to-myofibroblast differentiation in the diseased prostatic stroma. Mol. Endocrinol. 2011, 25, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Radermacher, K.A.; Wingler, K.; Langhauser, F.; Altenhofer, S.; Kleikers, P.; Hermans, J.J.; Hrabe de Angelis, M.; Kleinschnitz, C.; Schmidt, H.H. Neuroprotection after stroke by targeting Nox4 as a source of oxidative stress. Antioxid. Redox Signal. 2013, 18, 1418–1427. [Google Scholar] [CrossRef] [PubMed]
- Piera-Velazquez, S.; Jimenez, S.A. Role of cellular senescence and Nox4-mediated oxidative stress in systemic sclerosis pathogenesis. Curr. Rheumatol. Rep. 2015, 17, 473. [Google Scholar] [CrossRef] [PubMed]
- Dosoki, H.; Stegemann, A.; Taha, M.; Schnittler, H.; Luger, T.A.; Schroder, K.; Distler, J.H.; Kerkhoff, C.; Bohm, M. Targeting of NADPH oxidase in vitro and in vivo suppresses fibroblast activation and experimental skin fibrosis. Exp. Dermatol. 2017, 26, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Sanders, Y.Y.; Liu, H.; Liu, G.; Thannickal, V.J. Epigenetic mechanisms regulate NADPH oxidase-4 expression in cellular senescence. Free Radic. Biol. Med. 2015, 79, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Lener, B.; Koziel, R.; Pircher, H.; Hutter, E.; Greussing, R.; Herndler-Brandstetter, D.; Hermann, M.; Unterluggauer, H.; Jansen-Durr, P. The NADPH oxidase Nox4 restricts the replicative lifespan of human endothelial cells. Biochem. J. 2009, 423, 363–374. [Google Scholar] [CrossRef] [PubMed]
- Zidorn, C. Altitudinal variation of secondary metabolites in flowering heads of the Asteraceae: Trends and causes. Phytochem. Rev. 2010, 9, 197–203. [Google Scholar] [CrossRef]
- Turunen, M.; Latola, K. UV-B radiation and acclimation in timberline plants. Environ. Pollut. 2005, 137, 390–403. [Google Scholar] [CrossRef] [PubMed]
- Yaar, M.; Gilchrest, B.A. Photoageing: Mechanism, prevention and therapy. Br. J. Dermatol. 2007, 157, 874–887. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, N. Complete Herb Encyclopedia; Rebo: Noordwijkerhout, The Netherlands, 2003. [Google Scholar]
- Beer, A.M.; Wiebelitz, K.R.; Schmidt-Gayk, H. Lycopus europaeus (Gypsywort): Effects on the thyroidal parameters and symptoms associated with thyroid function. Phytomedicine 2008, 15, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Vonhoff, C.; Baumgartner, A.; Hegger, M.; Korte, B.; Biller, A.; Winterhoff, H. Extract of Lycopus europaeus L. reduces cardiac signs of hyperthyroidism in rats. Life Sci. 2006, 78, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Radulovic, N.; Denic, M.; Stojanovic-Radic, Z.; Skropeta, D. Fatty and Volatile Oils of the Gypsywort Lycopus europaeus L. and the Gaussian-Like Distribution of its Wax Alkanes. J. Am. Oil Chem. Soc. 2012, 89, 2165–2185. [Google Scholar] [CrossRef]
- Fialova, S.; Slobodnikova, L.; Veizerova, L.; GranCai, D. Lycopus europaeus: Phenolic fingerprint, antioxidant activity and antimicrobial effect on clinical Staphylococcus aureus strains. Nat. Prod. Res. 2015, 29, 2271–2274. [Google Scholar] [CrossRef] [PubMed]
- Bucar, F.; Kartnig, T.; Paschek, G.; Winker, E.; Schubert-Zsilavecz, M. Flavonoid glycosides from Lycopus europaeus. Planta Med. 1995, 61, 489. [Google Scholar] [CrossRef] [PubMed]
- Fecka, I.; Cisowski, W. Multiple gradient development TLC in analysis of complex phenolic acids from Lycopus europaeus. Chromatographia 1999, 49, 256–260. [Google Scholar] [CrossRef]
- Radulovic, N.; Denic, M.; Stojanovic-Radic, Z. Antimicrobial phenolic abietane diterpene from Lycopus europaeus L. (Lamiaceae). Bioorg. Med. Chem. Lett. 2010, 20, 4988–4991. [Google Scholar] [CrossRef] [PubMed]
- Sevindik, H.G.; Ozgen, U.; Atila, A.; Er, H.O.; Kazaz, C.; Duman, H. Phtytochemical studies and quantitative HPLC analysis of rosmarinic acid and luteolin 5-O-β-d-glucopyranoside on Thymus praecox subsp. Grossheimii var. Grossheimii. Chem. Pharm. Bull. 2015, 63, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Banfi, B.; Tirone, F.; Durussel, I.; Knisz, J.; Moskwa, P.; Molnar, G.Z.; Krause, K.-H.; Cox, J.A. Mechanism of Ca2+ Activation of the NADPH Oxidase 5 (Nox5). J. Biol. Chem. 2004, 279, 18583–18591. [Google Scholar] [CrossRef] [PubMed]
- Jaquet, V.; Scapozza, L.; Clark, R.A.; Krause, K.-H.; Lambeth, J.D. Small-Molecule Nox Inhibitors: ROS-Generating NADPH Oxidases as Therapeutic Targets. Antioxid. Redox Signal. 2009, 11, 2535–2552. [Google Scholar] [CrossRef] [PubMed]
- Stoeckl, P.; Huetter, E.; Zwerschke, W.; Jansen-Duerr, P. Sustained inhibition of oxidative phosphorylation impairs cell proliferation and induces premature senescence in human fibroblasts. Exp. Gerontol. 2006, 41, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Al-Sereiti, M.R.; Abu-Amer, K.M.; Sen, P. Pharmacology of rosemary (Rosmarinus officinalis Linn.) and its therapeutic potentials. Indian J. Exp. Biol. 1999, 37, 124–130. [Google Scholar] [PubMed]
- Swiatek, L.; Grabias, B.; Rozga, M. Phenolic acids and coumarins in Lithospermum arvense L. and Lycopus europaeus L. Herb. Pol. 1987, 33, 87–97. [Google Scholar]
- Shekarchi, M.; Hajimehdipoor, H.; Saeidnia, S.; Gohari, A.R.; Hamedani, M.P. Comparative study of rosmarinic acid content in some plants of Labiatae family. Pharmacogn. Mag. 2012, 8, 37–41. [Google Scholar] [PubMed]
- Fuhrman, B.; Volkova, N.; Rosenblat, M.; Aviram, M. Lycopene synergistically inhibits LDL oxidation in combination with vitamin E, glabridin, rosmarinic acid, carnosic acid, or garlic. Antioxid. Redox Signal. 2000, 2, 491–506. [Google Scholar] [CrossRef] [PubMed]
- Fadel, O.; El Kirat, K.; Morandat, S. The natural antioxidant rosmarinic acid spontaneously penetrates membranes to inhibit lipid peroxidation in situ. Biochim. Biophys. Acta Biomembr. 2011, 1808, 2973–2980. [Google Scholar] [CrossRef] [PubMed]
- Osakabe, N.; Yasuda, A.; Natsume, M.; Yoshikawa, T. Rosmarinic acid inhibits epidermal inflammatory responses: Anticarcinogenic effect of Perilla frutescens extract in the murine two-stage skin model. Carcinogenesis 2004, 25, 549–557. [Google Scholar] [CrossRef] [PubMed]
- Takano, H.; Osakabe, N.; Sanbongi, C.; Yanagisawa, R.; Inoue, K.I.; Yasuda, A.; Natsume, M.; Baba, S.; Ichiishi, E.I.; Yoshikawa, T. Extract of Perilla frutescens enriched for rosmarinic acid, a polyphenolic phytochemical, inhibits seasonal allergic rhinoconjunctivitis in humans. Exp. Biol. Med. 2004, 229, 247–254. [Google Scholar] [CrossRef]
- Dubois, M.; Bailly, F.; Mbemba, G.; Mouscadet, J.-F.; Debyser, Z.; Witvrouw, M.; Cotelle, P. Reaction of Rosmarinic Acid with Nitrite Ions in Acidic Conditions: Discovery of Nitro- and Dinitrorosmarinic Acids as New Anti-HIV-1 Agents. J. Med. Chem. 2008, 51, 2575–2579. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.; Simmonds, M.S. Rosmarinic acid. Phytochemistry 2003, 62, 121–125. [Google Scholar] [CrossRef]
- Takeda, H.; Tsuji, M.; Inazu, M.; Egashira, T.; Matsumiya, T. Rosmarinic acid and caffeic acid produce antidepressive-like effect in the forced swimming test in mice. Eur. J. Pharmacol. 2002, 449, 261–267. [Google Scholar] [CrossRef]
- Huang, S.S.; Zheng, R.L. Rosmarinic acid inhibits angiogenesis and its mechanism of action in vitro. Cancer Lett. 2006, 239, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Ohto, Y.; Murakami, A.; Ohigashi, H. Superoxide Scavenging Activity of Rosmarinic Acid from Perilla frutescens Britton Var. acuta f. viridis. J. Agric. Food Chem. 1998, 46, 4545–4550. [Google Scholar] [CrossRef]
- Validation of Analytical Procedures: Text and Methodology. Available online: https://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm265700.htm (accessed on 13 September 2017).
- Arndt-Jovin, D.J.; Jovin, T.M. Analysis and sorting of living cells according to deoxyribonucleic acid content. J. Histochem. Cytochem. 1977, 25, 585–589. [Google Scholar] [CrossRef] [PubMed]
- Latt, S.A.; Loveday, K.S. Characterization of sister chromatid exchange induction by 8-methoxypsoralen plus near uv light. Cytogenet. Genome Res. 1978, 21, 184–200. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Not available. |





| Plant Part | Extraction Solvent | Extract Yield (wt %) | Nox4-Dependent Chemiluminescence Quenching (%) at 125 µg/mL | Cell Viability (%) at 125 µg/mL | Rosmarinic Acid Content (wt %) |
|---|---|---|---|---|---|
| subaerial parts | ethyl acetate | 0.98 | 30 ± 8.90 | 63 ± 0.043 | 1.02 ± 0.003 |
| subaerial parts | methanol 1 | 3.60 | 81 ± 5.07 | 91 ± 0.004 | 10.14 ± 0.120 |
| aerial parts | ethyl acetate | 2.50 | 98 ± 0.41 | 15 ± 0.003 | 0.26 ± 0.001 |
| aerial parts | methanol 1 | 3.52 | 79 ± 4.77 | 69 ± 0.010 | 7.50 ± 0.140 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Revoltella, S.; Baraldo, G.; Waltenberger, B.; Schwaiger, S.; Kofler, P.; Moesslacher, J.; Huber-Seidel, A.; Pagitz, K.; Kohl, R.; Jansen-Duerr, P.; et al. Identification of the NADPH Oxidase 4 Inhibiting Principle of Lycopus europaeus. Molecules 2018, 23, 653. https://doi.org/10.3390/molecules23030653
Revoltella S, Baraldo G, Waltenberger B, Schwaiger S, Kofler P, Moesslacher J, Huber-Seidel A, Pagitz K, Kohl R, Jansen-Duerr P, et al. Identification of the NADPH Oxidase 4 Inhibiting Principle of Lycopus europaeus. Molecules. 2018; 23(3):653. https://doi.org/10.3390/molecules23030653
Chicago/Turabian StyleRevoltella, Silvia, Giorgia Baraldo, Birgit Waltenberger, Stefan Schwaiger, Philipp Kofler, Julia Moesslacher, Astrid Huber-Seidel, Konrad Pagitz, Roland Kohl, Pidder Jansen-Duerr, and et al. 2018. "Identification of the NADPH Oxidase 4 Inhibiting Principle of Lycopus europaeus" Molecules 23, no. 3: 653. https://doi.org/10.3390/molecules23030653
APA StyleRevoltella, S., Baraldo, G., Waltenberger, B., Schwaiger, S., Kofler, P., Moesslacher, J., Huber-Seidel, A., Pagitz, K., Kohl, R., Jansen-Duerr, P., & Stuppner, H. (2018). Identification of the NADPH Oxidase 4 Inhibiting Principle of Lycopus europaeus. Molecules, 23(3), 653. https://doi.org/10.3390/molecules23030653