A Novel Approach for Microbial Synthesis of Enantiomerically Pure Whisky Lactones Based on Solid-State Fermentation
Abstract
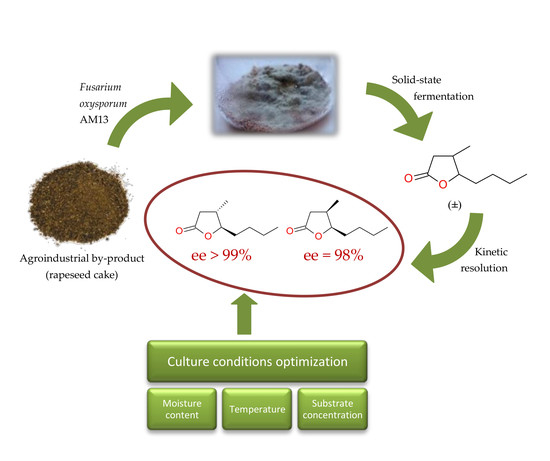
:1. Introduction
2. Results
3. Materials and Methods
3.1. Materials
3.2. Solid-State Fermentation
3.3. Biotransformation Process
3.4. Optimization Process
3.5. Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dumitriu, G.D.; López de Lerma, N.; Zamfir, C.I.; Cotea, V.V.; Peinado, R.A. Volatile and phenolic composition of red wines subjected to aging in oak cask of different toast degree during two periods of time. LWT-Food Sci. Technol. 2017, 86, 643–651. [Google Scholar] [CrossRef]
- Maga, J.A. Oak lactones in alcoholic beverages. Food Rev. Int. 1996, 12, 105–130. [Google Scholar] [CrossRef]
- Günther, C.; Mosandl, A. Stereoisomere Aromastoffe, XII. 3-Methyl-4-octanolid—Quercuslacton, Whiskylacton-Struktur und Eigenschaften der Stereoisomeren. Liebigs Ann. Chem. 1986, 1986, 2112–2122. [Google Scholar] [CrossRef]
- Abbott, N.; Puech, J.L.; Bayonove, C.; Baumes, R. Determination of the aroma threshold of the cis and trans racemic forms of β-methyl-γ-octalactone by gas chromatography—Sniffing analysis. Am. J. Enol. Vitic. 1995, 46, 292–294. [Google Scholar]
- Sefton, M.A.; Francis, I.L.; Pocock, K.F.; Williams, P.J. The influence of natural seasoning on the concentrations of eugenol, vanillin, and cis-beta-methyl-gamma-octalactone and trans-beta-methyl-gamma-octalactone extracted from French and American oakwood. Sci. Aliments 1993, 13, 629–643. [Google Scholar]
- Jefford, C.W.; Sledeski, A.W.; Boukouvalas, J. Synthesis of cis and trans whisky and cognac lactones by the regiocontrolled alkylation of 2-(trimethylsiloxy)furan. Helv. Chim. Acta 1989, 72, 1362–1370. [Google Scholar] [CrossRef]
- Nishikori, H.; Ito, K.; Katsuki, T. A short-step synthesis of trans-whisky lactone by an asymmetric Michael reaction. Tetrahedron Asymmetry 1998, 9, 1165–1170. [Google Scholar] [CrossRef]
- Jiang, X.; Fu, C.; Ma, S. A concise synthesis of (−)- and (+)- trans -Whisky lactones. Eur. J. Org. Chem. 2010, 2010, 687–693. [Google Scholar] [CrossRef]
- Marina, J.P.; de la Pradilla, R.F. Stereospecific synthesis of y-butyrolactones from acyclic vinyl sulfoxides: an asymmetric synthesis of optically pure oak lactones. Tetrahedron Lett. 1985, 26, 5381–5384. [Google Scholar] [CrossRef]
- Pisani, L.; Superchi, S.; D’Elia, A.; Scafato, P.; Rosini, C. Synthetic approach toward cis-disubstituted γ- and δ-lactones through enantioselective dialkylzinc addition to aldehydes: Application to the synthesis of optically active flavors and fragrances. Tetrahedron 2012, 68, 5779–5784. [Google Scholar] [CrossRef]
- Etschmann, M.; Bluemke, W.; Sell, D.; Schrader, J. Biotechnological production of 2-phenylethanol. Appl. Microbiol. Biotechnol. 2002, 59, 1–8. [Google Scholar] [PubMed]
- Boratyński, F.; Smuga, M.; Wawrzeńczyk, C. Lactones 42. Stereoselective enzymatic/microbial synthesis of optically active isomers of whisky lactone. Food Chem. 2013, 141, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Felluga, F.; Forzato, C.; Ghelfi, F.; Nitti, P.; Pitacco, G.; Pagnoni, U.M.; Roncaglia, F. Atom transfer radical cyclization (ATRC) applied to a chemoenzymatic synthesis of Quercus lactones. Tetrahedron Asymmetry 2007, 18, 527–536. [Google Scholar] [CrossRef]
- Ramachandran, S.; Singh, S.K.; Larroche, C.; Soccol, C.R.; Pandey, A. Oil cakes and their biotechnological applications-A review. Bioresour. Technol. 2007, 98, 2000–2009. [Google Scholar] [CrossRef] [PubMed]
- Gutarra, M.L.E.; Cavalcanti, E.D.C.; Castilho, L.R.; Freire, D.M.G.; Sant’Anna, G.L. Lipase production by solid-state fermentation: cultivation conditions and operation of tray and packed-bed bioreactors. Appl. Biochem. Biotechnol. 2005. [Google Scholar] [CrossRef]
- Palma, M.B.; Pinto, A.L.; Gombert, A.K.; Seitz, K.H.; Kivatinitz, S.C.; Castilho, L.R.; Freire, D.M. Lipase production by Penicillium restrictum using solid waste of industrial babassu oil production as substrate. Appl. Biochem. Biotechnol. 2000. [Google Scholar] [CrossRef]
- Santhosh Kumar, D. Fungal lipase production by solid state fermentation—An overview. J. Anal. Bioanal. Tech. 2015, 6, 1–10. [Google Scholar] [CrossRef]
- Couto, S.R.; Sanromán, M.Á. Application of solid-state fermentation to food industry-A review. J. Food Eng. 2006, 76, 291–302. [Google Scholar] [CrossRef]
- Damaso, M.C.T.; Passianoto, M.A.; De Freitas, S.C.; Freire, D.M.G.; Lago, R.C.A.; Couri, S. Utilization of agroindustrial residues for lipase production by solid-state fermentation. Braz. J. Microbiol. 2008, 39, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Kapilan, R. Solid state fermentation for microbial products: A review. Appl. Sci. Res. 2015, 7, 21–25. [Google Scholar]
- Papagianni, M. An evaluation of the proteolytic and lipolytic potential of Penicillium spp. isolated from traditional greek sausages in submerged fermentation. Appl. Biochem. Biotechnol. 2014, 172, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Freitas, A.C.; Castro, R.J.S.; Fontenele, M.A.; Egito, A.S.; Farinas, C.S.; Pinto, G.A.S. Canola cake as a potential substrate for proteolytic enzymes production by a selected strain of Aspergillus oryzae: Selection of process conditions and product characterization. ISRN Microbiol. 2013, 2013, 369082. [Google Scholar] [CrossRef] [PubMed]
- Bhargav, S.; Panda, B.P.; Ali, M.; Javed, S. Solid-state fermentation systems-an overview. Crit. Rev. Biotechnol. 2008, 25, 1–30. [Google Scholar]
- Pandey, A. Solid-state fermentation. Biochem. Eng. J. 2003, 13, 81–84. [Google Scholar] [CrossRef]
- Hölker, U.; Lenz, J. Solid-state fermentation—Are there any biotechnological advantages? Curr. Opin. Microbiol. 2005, 8, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Lomascolo, A.; Uzan-Boukhris, E.; Sigoillot, J.C.; Fine, F. Rapeseed and sunflower meal: A review on biotechnology status and challenges. Appl. Microbiol. Biotechnol. 2012, 95, 1105–1114. [Google Scholar] [CrossRef] [PubMed]
- Sugumaran, K.R.; Jothi, P.; Ponnusami, V. Bioconversion of industrial solid waste—Cassava bagasse for pullulan production in solid state fermentation. Carbohydr. Polym. 2014, 99, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Madrera, R.R.; Pando, R. Production and characterization of aroma compounds from apple pomace by solid-state fermentation with selected yeasts. LWT Food Sci. Technol. 2015, 64, 1342–1353. [Google Scholar] [CrossRef]
- Ibrahim, D.; Che, I. Kinetic resolutions with novel, highly enantioselective fungal lipases produced by solid state fermentation. J. Mol. Catal. B Enzym. 2006, 39, 141–148. [Google Scholar]
- Salihu, A.; Alam, Z.; Abdulkarim, M.I.; Salleh, H.M. Resources, conservation and recycling lipase production: An insight in the utilization of renewable agricultural residues. Resourc. Conserv. Recycl. 2012, 58, 36–44. [Google Scholar] [CrossRef]
- Brenna, E.; Fuganti, C.; Gatti, F.G.; Serra, S. Biocatalytic methods for the synthesis of enantioenriched odor active compounds. Chem. Rev. 2011, 111, 4036–4072. [Google Scholar] [CrossRef] [PubMed]
- Boratyński, F.; Szczepańska, E.; Grudniewska, A.; Olejniczak, T. Microbial kinetic resolution of aroma compounds using solid-state fermentation. Catalysts 2018, 8, 28. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |



| Strain | Time (Days) | trans/cis Ratio (%) | trans-(+)-(4S,5R) ee a (%) | cis-(+)-(4R,5R) ee a (%) |
|---|---|---|---|---|
| Aspergillus sp. AM31 | 3 | 67/33 | 0 | 0 |
| 6 | 59/41 | 38 | 28 | |
| Fusarium culmorum AM9 | 3 | 48/52 | 12 | 14 |
| 6 | 55/45 | 16 | 12 | |
| Fusarium equiseti AM15 | 3 | 50/50 | 24 b | 18 |
| 6 | 50/50 | 24 b | 18 | |
| Fusarium oxysporum AM13 | 3 | 56/44 | 0 | 0 |
| 6 | 56/44 | 56 | 60 | |
| Papularia rosea AM17 | 3 | 38/62 | 46 | 26 |
| 6 | 33/67 | 70 | 42 | |
| Penicillum camemberti AM83 | 3 | 77/23 | 32 | 0 |
| 6 | 57/43 | 30 | 10 | |
| Penicillium chrysogenum AM112 | 3 | 77/23 | 24 | 20 |
| 6 | 53/47 | 42 | 14 | |
| Penicillium notatum AM904 | 3 | 70/30 | 0 | 0 |
| 6 | 58/42 | 52 | 12 | |
| Penicillium vermiculatum AM30 | 3 | -/- | - | - |
| 6 | -/- | - | - | |
| Pycnidiella resinae AM50 | 3 | 41/59 | 23 | 0 |
| 6 | 27/73 | 40 | 0 |
| Strain | Temperature (°C) | Moisture (%) | 2 Days | 5 Days | ||||
|---|---|---|---|---|---|---|---|---|
| trans/cis Ratio (%) | trans-(+)-(4S,5R) ee a (%) | cis-(+)-(4R,5R) ee a (%) | trans/cis Ratio (%) | trans-(+)-(4S,5R) ee a (%) | cis-(+)-(4R,5R) ee a (%) | |||
| Fusarium oxysporum AM13 | 23 | 40 | 53/47 | 28 | 24 | 56/44 | 68 | 50 |
| 60 | 55/45 | 58 | 40 | 61/39 | 88 | 64 | ||
| 80 | 56/44 | 68 | 38 | 59/41 | 88 | 54 | ||
| 100 | 56/44 | 56 | 38 | 60/40 | 90 | 60 | ||
| 28 | 40 | 56/44 | 56 | 42 | 62/38 | 84 | 76 | |
| 60 | 64/36 | 82 | 44 | 62/38 | 76 | 38 | ||
| 80 | 70/30 | 24 | 16 | 68/32 | 38 | 16 | ||
| 100 | 71/29 | 18 | 12 | 69/31 | 20 | 18 | ||
| 33 | 40 | 60/40 | 48 | 44 | 60/40 | 58 | 66 | |
| 60 | 66/34 | 70 | 66 | 61/39 | 98 | 82 | ||
| 80 | 63/37 | 72 | 60 | 68/32 | 88 | 74 | ||
| 100 | 62/38 | 44 | 40 | 65/35 | 78 | 54 | ||
| Papularia rosea AM17 | 23 | 40 | 51/49 | 30 | 26 | 34/66 | 78 | 36 |
| 60 | 60/40 | 26 | 14 | 62/38 | 52 | 24 | ||
| 80 | 73/27 | 10 | 10 | 70/30 | 18 | 16 | ||
| 100 | 73/27 | 8 | 14 | 69/31 | 22 | 16 | ||
| 28 | 40 | 40/60 | 40 | 26 | 38/62 | 52 | 30 | |
| 60 | 43/57 | 86 | 32 | 38/62 | 86 | 34 | ||
| 80 | 57/43 | 36 | 28 | 54/46 | 52 | 34 | ||
| 100 | 68/32 | 8 | 6 | - | - | - | ||
| 33 | 40 | 35/65 | 72 | 30 | - | - | - | |
| 60 | 53/47 | 60 | 10 | - | - | - | ||
| 80 | 51/49 | 56 | 16 | - | - | - | ||
| 100 | 59/41 | 40 | 14 | - | - | - | ||
| Conditions | Parameters | Enantiomeric Excess | |||
|---|---|---|---|---|---|
| Temperature (°C) | Moisture (%) | Concentration of Substrate (mg/g of Oil Cake) | Trans-(+)-(4S,5R) (%) | Cis-(+)-(4R,5R) (%) | |
| Initial | 23 | 60 | 1.5 | 56 | 60 |
| Optimized | 33 | 60 | 3 | > 99 | 98 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boratyński, F.; Szczepańska, E.; Grudniewska, A.; Skalny, B.; Olejniczak, T. A Novel Approach for Microbial Synthesis of Enantiomerically Pure Whisky Lactones Based on Solid-State Fermentation. Molecules 2018, 23, 659. https://doi.org/10.3390/molecules23030659
Boratyński F, Szczepańska E, Grudniewska A, Skalny B, Olejniczak T. A Novel Approach for Microbial Synthesis of Enantiomerically Pure Whisky Lactones Based on Solid-State Fermentation. Molecules. 2018; 23(3):659. https://doi.org/10.3390/molecules23030659
Chicago/Turabian StyleBoratyński, Filip, Ewa Szczepańska, Aleksandra Grudniewska, Bartłomiej Skalny, and Teresa Olejniczak. 2018. "A Novel Approach for Microbial Synthesis of Enantiomerically Pure Whisky Lactones Based on Solid-State Fermentation" Molecules 23, no. 3: 659. https://doi.org/10.3390/molecules23030659
APA StyleBoratyński, F., Szczepańska, E., Grudniewska, A., Skalny, B., & Olejniczak, T. (2018). A Novel Approach for Microbial Synthesis of Enantiomerically Pure Whisky Lactones Based on Solid-State Fermentation. Molecules, 23(3), 659. https://doi.org/10.3390/molecules23030659