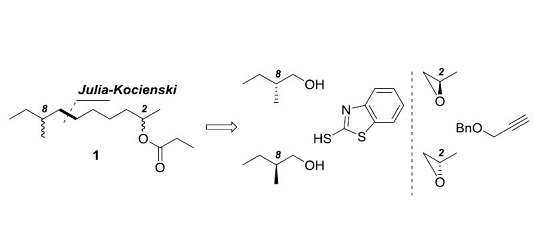
3.1.2. General Procedure for the Synthesis of Compounds
(S)-2-(2-Methylbutylthio)benzo[d]thiazole ((S)-8). To a stirred solution of (S)-5 (0.500 g, 5.67 mmol) in THF (30.0 mL) at 0 °C was added Ph3P (1.785 g, 6.80 mmol) and 6 (1.138 g, 6.80 mmol). DIAD (1.375 g, 6.80 mmol) was added, and the resulting mixture was allowed to warm to room temperature and stirred for 4 h. After removal of solvent, the residue was purified by flash chromatography on silica gel (hexanes:ethyl acetate = 50:1) to afford compound (S)-8 as a yellow oil (1.333 g, 99%). = +22.0 (c = 0.5, CHCl3). 1H-NMR (500 MHz, CDCl3) δ 7.86 (d, J = 8.0 Hz, 1H), 7.74 (d, J = 7.5 Hz, 1H), 7.42–7.39 (m, 1H), 7.30–7.26 (m, 1H), 3.41 (dd, J = 6.0, 13.0 Hz, 1H), 3.21 (dd, J = 7.5, 13.0 Hz, 1H), 1.90–1.84 (m, 1H), 1.62–1.54 (m, 1H), 1.38–1.29 (m, 1H), 1.07 (d, J = 7.0 Hz, 3H), 0.96 (t, J = 7.5 Hz, 3H); 13C-NMR (125 MHz, CDCl3) δ 167.68, 153.32, 135.12, 125.92, 124.02, 121.38, 120.83, 40.32, 34.81, 28.69, 18.83, 11.27; HRMS (ESI) calcd for C12H16NS2+ [M + H]+: 238.0718, found: 238.0719.
HPLC analysis: Daicel Chiralcel OD-H column; hexane/i-propanol = 98:2, 0.7 mL/min, λ = 220 nm; tR (major) = 9.31 min, tR (minor) = 9.85 min; 99:1 er.
(S)-2-(2-Methylbutylsulfonyl)benzo[d]thiazole ((S)-3). To a solution of (S)-8 (2.090 g, 8.80 mmol) in CH2Cl2 (90.0 mL) at room temperature was added m-CPBA (85% purity, 8.933 g, 44.00 mmol). Upon stirring overnight, the reaction mixture was quenched with saturated aqueous Na2S2O3 (10.0 mL) and NaHCO3 (20.0 mL), extracted with CH2Cl2, washed with brine, dried over anhydrous Na2SO4, concentrated, and purified by flash chromatography on silica gel (hexanes:ethyl acetate = 30:1) to give compound (S)-3 as a pale yellow oil (2.229 g, 94%). = +14.0 (c = 0.5, CHCl3). 1H-NMR (500 MHz, CDCl3) δ 8.21 (d, J = 8.0 Hz, 1H), δ 8.01 (d, J = 7.5 Hz, 1H), 7.65–7.57 (m, 2H), 3.55 (dd, J = 5.0, 14.5 Hz, 1H), 3.35 (dd, J = 8, 14.0 Hz, 1H), 2.28–2.19 (m, 1H), 1.59–1.51 (m, 1H), 1.43–1.34 (m, 1H), 1.13 (d, J = 7.0 Hz, 3H), 0.90 (t, J = 7.5 Hz, 3H); 13C-NMR (125 MHz, CDCl3) δ 166.80, 152.69, 136.72, 127.94, 127.59, 125.39, 122.33, 60.43, 29.92, 29.33, 19.30, 10.67; HRMS (ESI) calcd for C12H15NO2S2Na+ [M + Na]+: 292.0441, found: 292.0433.
(R)-4-Benzyl-3-butyryloxazolidin-2-one (11). To a stirred solution of 9 (10.000 g, 56.43 mmol) in THF (120.0 mL) at −78 °C under Ar was added n-BuLi (1.6 M in hexanes, 42.3 mL, 67.68 mmol) over 30 min. After stirred for 30 min, n-butyryl chloride (10) (8.6 mL, 84.70 mmol) was added dropwise. The reaction mixture was allowed to warmed to room temperature and stirred overnight, quenched with saturated aqueous NH4Cl, extracted with ethyl acetate, washed with brine, dried over anhydrous Na2SO4, concentrated, and purified by flash chromatography on silica gel (hexanes:ethyl acetate = 5:1) to give 11 as a colorless oil (13.950 g, 100%). = −69.0 (c = 0.1, CHCl3). 1H-NMR (500 MHz, CDCl3) δ 7.35–7.21 (m, 5H), 4.69–4.66 (m, 1H), 4.21–4.15 (m, 2H), 3.31–3.28 (m, 1H), 2.99–2.85 (m, 2H), 2.77 (dd, J = 9.5, 13.0 Hz, 1H), 1.75–1.71 (m, 2H), 1.01 (t, J = 7.5 Hz, 3H); 13C-NMR (125 MHz, CDCl3) δ 173.17, 153.42, 135.29, 129.36, 128.88, 127.27, 66.10, 55.07, 37.88, 37.32, 17.65, 13.62.
(R)-4-Benzyl-3-((R)-2-methylbutanoyl) oxazolidin-2-one (12). To a stirred solution of 11 (14.270 g, 57.70 mmol) in THF (280.0 mL) at −78 °C under Ar was added NaHMDS (2.0 M in THF, 58.0 mL, 116.00 mmol) dropwise. After 30 min, MeI (18.0 mL, 288.40 mmol) was added dropwise. Upon stirring at −78 °C for 2 h, the reaction was quenched with saturated aqueous NH4Cl, extracted with ethyl acetate, washed with brine, dried over anhydrous Na2SO4, concentrated, and purified by flash chromatography on silica gel (hexanes:ethyl acetate = 5:1) to give 12 as a pale yellow oil (12.034 g, 80%). = −75.9 (c = 0.1, CHCl3). 1H-NMR (500 MHz, CDCl3) δ 7.34–7.21 (m, 5H), 4.70–4.67 (m, 1H), 4.21–4.16 (m, 2H), 3.66–3.62 (m, 1H), 3.29–3.26 (m, 1H), 2.77 (dd, J = 10.0, 13.5 Hz, 1H), 1.82–1.73 (m, 1H), 1.52–1.43 (m, 1H), 1.22 (d, J = 6.5 Hz, 3H), 0.93 (t, J = 7.0 Hz, 3H); 13C-NMR (125 MHz, CDCl3) δ 177.13, 153.05, 135.32, 129.41, 128.88, 127.28, 65.97, 55.30, 39.13, 37.87, 26.36, 16.85, 11.60.
(R)-2-Methylbutan-1-ol ((R)-5). To a solution of 12 (8.571 g, 32.8 mmol) in Et2O/MeOH (100.0 mL/3.0 mL) at −30 °C under Ar was added LiBH4 (2.0 M in THF, 11.5 mL, 23.0 mmol) dropwise. Upon stirring at −30 °C for 30 min, the reaction mixture was moved to an ice bath and stirred overnight, quenched with 10% aqueous NaOH, extracted with Et2O, dried over anhydrous Na2SO4, concentrated, and purified by flash chromatography on silica gel (n-pentane: Et2O = 5:1) to give (R)-5 as a colorless oil (2.660 g, 92%). = +5.0 (c = 1.0, CHCl3). 1H-NMR (500 MHz, CDCl3) δ 3.54–3.47 (m, 1H), 3.47–3.38 (m, 1H), 1.60–1.50 (m, 1H), 1.50–1.40 (m, 1H), 1.27–1.19 (m, 1H), 1.18–1.09 (m, 1H), 0.91 (t, J = 7.4 Hz, 3H), 0.91 (d, J = 6.7 Hz, 3H); 13C-NMR (500 MHz, CDCl3) δ 67.93, 37.31, 25.70, 16.04, 11.26.
(R)-2-(2-Methylbutylthio)benzo[d]thiazole ((R)-8). To a stirred solution of (R)-5 (0.635 g, 7.20 mmol) in THF (70.0 mL) at 0 °C was added Ph3P (2.267 g, 8.64 mmol) and 6 (1.446 g, 8.64 mmol). DIAD (1.734 g, 8.64 mmol) was added, and the resulting mixture was allowed to warm to room temperature and stirred for 4 h. After removal of solvent, the residue was purified by flash chromatography on silica gel (hexanes:ethyl acetate = 50:1) to afford compound (R)-8 as a yellow oil (1.400 g, 82%). = −26.0 (c = 0.3, CHCl3). 1H-NMR (500 MHz, CDCl3) δ 7.86 (d, J = 8.0 Hz, 1H), 7.74 (d, J = 8.0 Hz, 1H), 7.40 (t, J = 8.0 Hz, 1H), 7.28 (dt, J = 1.0, 8.5 Hz, 1H), 3.41 (dd, J = 6.0, 13.0 Hz, 1H), 3.21 (dd, J = 7.5, 13.0 Hz, 1H), 1.90–1.84 (m, 1H), 1.62–1.54 (m, 1H), 1.38–1.29 (m, 1H), 1.07 (d, J = 6.5 Hz, 3H), 0.96 (t, J = 7.5 Hz, 3H); 13C-NMR (125 MHz, CDCl3) δ 167.73, 153.35, 135.16, 125.96, 124.06, 121.42, 120.87, 40.36, 34.84, 28.72, 18.86, 11.29.
HPLC analysis: Daicel Chiralcel OD-H column; hexane/i-propanol = 98:2, 0.7 mL/min, λ = 220 nm; tR (major) = 9.79 min, tR (minor) = 9.28 min; 93:7 er.
(R)-2-(2-Methylbutylsulfonyl)benzo[d]thiazole ((R)-3). To a solution of (R)-8 (1.300 g, 5.48 mmol) in CH2Cl2 (55.0 mL) at room temperature was added m-CPBA (85% purity, 5.563 g, 27.40 mmol). Upon stirring overnight, the reaction mixture was quenched with saturated aqueous Na2S2O3 (10.0 mL) and NaHCO3 (20.0 mL), extracted with CH2Cl2, washed with brine, dried over anhydrous Na2SO4, concentrated, and purified by flash chromatography on silica gel (hexanes:ethyl acetate = 30:1) to give compound (R)-3 as a pale yellow oil (1.357 g, 92%). = −17.0 (c = 0.4, CHCl3). 1H-NMR (500 MHz, CDCl3) δ 8.20 (d, J = 8.0 Hz, 1H), δ 8.00 (d, J = 7.5 Hz, 1H), 7.65–7.57 (m, 2H), 3.55 (dd, J = 4.5, 14.0 Hz, 1H), 3.35 (dd, J = 8, 14.5 Hz, 1H), 2.26–2.20 (m, 1H), 1.58–1.51 (m, 1H), 1.43–1.34 (m, 1H), 1.13 (d, J = 6.5 Hz, 3H), 0.90 (t, J = 7.5 Hz, 3H); 13C-NMR (125 MHz, CDCl3) δ 166.81, 152.69, 136.73, 127.94, 127.59, 125.41, 122.33, 60.41, 29.93, 29.34, 19.31, 10.68.
(S)-6-(Benzyloxy)hex-4-yn-2-ol ((S)-14). To a stirred solution of 13 (5.350 g, 36.60 mmol) in THF (240.0 mL) at −78 °C under Ar was added n-BuLi (2.5 M in hexanes, 16.0 mL, 40.0 mmol). After 30 min, BF3.Et2O (5.0 mL, 39.90 mmol) was added, followed by (S)-2-methyloxirane ((S)-7) (1.933 g, 33.28 mmol). Upon stirring at −78 °C overnight, the reaction was quenched with saturated aqueous NH4Cl, extracted with ethyl acetate, washed with brine, dried over anhydrous Na2SO4, concentrated, and purified by flash chromatography on silica gel (hexanes:ethyl acetate = 10:1) to give (S)-14 as a pale yellow oil (5.303 g, 78%). = +8.0 (c = 0.2, CHCl3). 1H-NMR (500 MHz, CDCl3) 7.36–7.28 (m, 5H), 4.59 (s, 2H), 4.18 (d, J = 2.5 Hz, 2H), 3.99–3.93 (m, 1H), 2.48–2.36 (m, 2H), 2.06–1.85 (m, 1H), 1.27 (d, J = 6.0 Hz, 3H); 13C-NMR (125 MHz, CDCl3) δ 137.46, 128.41, 128.03, 127.83, 83.32, 78.51, 71.58, 66.33, 57.63, 29.36, 22.35.
(S)-6-(Benzyloxy)hex-4-yn-2-yl propionate ((S)-15). To a solution of (S)-14 (5.000 g, 24.48 mmol) in CH2Cl2 (120.0 mL) at 0 °C was added propionic acid (2.720 g, 36.72 mmol), DCC (7.576 g, 36.72 mmol), and DMAP (0.300 g, 2.45 mmol). The reaction mixture was stirred overnight, diluted with water, washed with saturated aqueous NaHCO3, extracted with CH2Cl2, washed with brine, dried over anhydrous Na2SO4, concentrated, and purified by flash chromatography on silica gel (hexanes:ethyl acetate = 50:1) to give (S)-15 as a pale yellow oil (6.245 g, 98%). = −32.0 (c = 0.3, CHCl3). 1H-NMR (500 MHz, CDCl3) 7.36–7.28 (m, 5H), 5.05–4.99 (m, 1H), 4.58 (s, 2H), 4.16 (d, J = 2.0 Hz, 2H), 2.57–2.47 (m, 2H), 2.31 (q, J = 7.5 Hz, 2H), 1.34 (d, J = 6.5 Hz, 3H), 1.13 (t, J = 7.5 Hz, 3H); 13C-NMR (125 MHz, CDCl3) δ 173.82, 173.55, 128.40, 128.06, 127.80, 82.44, 78.06, 71.37, 68.52, 57.54, 27.78, 25.95, 19.23, 9.11; HRMS (ESI) calcd for C16H20NO3Na+ [M + Na]+: 283.1310, found: 283.1307.
(S)-6-Hydroxyhexan-2-yl propionate ((S)-16). To a stirred solution of (S)-15 (1.200 g, 4.61 mmol) in MeOH (46.0 mL) was added Pd/C (10%, 0.200 g). The flask was evacuated and refilled with H2 (balloon). This process was repeated for 3 times. Upon stirring at room temperature for 24 h, the reaction mixture was filtered, concentrated, and purified by flash chromatography on silica gel (hexanes:ethyl acetate = 10:1) to give (S)-16 as a colorless oil (0.747 g, 93%). = +4.0 (c = 0.8, CHCl3). 1H-NMR (500 MHz, CDCl3) δ 4.94–4.88 (m, 1H), 3.63 (d, J = 6.5 Hz, 2H), 2.29 (q, J = 7.5 Hz, 2H), 1.64–1.35 (m, 7H), 1.20 (d, J = 6.5 Hz, 3H), 1.12 (t, J = 7.5 Hz, 3H); 13C-NMR (125 MHz, CDCl3) δ 174.22, 70.57, 62.70, 35.69, 32.47, 27.91, 21.64, 19.95, 9.18; HRMS (ESI) calcd for C9H18O3Na+ [M + Na]+: 197.1154, found: 197.1147.
(S)-6-Oxohexan-2-yl propionate ((S)-4). To a stirred solution of (S)-16 (0.600 g, 3.44 mmol) in CH2Cl2 (35.0 mL) was added silica gel (1.100 g) and PCC (1.078 g, 5.0 mmol). Upon stirring at room temperature for 12 h, the reaction mixture was precipitated by adding petroleum ether, filtered through a pad of Celite, concentrated, and purified by flash chromatography on silica gel (hexanes:ethyl acetate = 50:1) to give (S)-4 as a colorless oil (0.458 g, 77%). = +5.3 (c = 0.8, CHCl3). 1H-NMR (500 MHz, CDCl3) δ 9.74 (t, J = 1.5 Hz, 1H), 4.93–4.87 (m, 1H), 2.46–2.42 (m, 2H), 2.28 (q, J = 7.5 Hz, 2H), 1.70–1.48 (m, 4H), 1.20 (d, J = 6.0 Hz, 3H), 1.11 (t, J = 7.5 Hz, 3H); 13C-NMR (125 MHz, CDCl3) δ 201.93, 174.06, 70.06, 43.45, 35.19, 27.84, 19.86, 17.88, 9.12; HRMS (ESI) calcd for C18H32O6Na+ [2M + Na]+: 367.2096, found: 367.2082.
(R)-6-(Benzyloxy)hex-4-yn-2-yl propionate ((R)-14). To a stirred solution of 13 (6.493 g, 44.41 mmol) in THF (250.0 mL) at −78 °C under Ar was added n-BuLi (2.5 M in hexanes, 21.3 mL, 53.25 mmol). After 30 min, BF3.Et2O (6.7 mL, 53.47 mmol) was added, followed by (R)-2-methyloxirane ((R)-7) (2.580 g, 44.41 mmol). Upon stirring at −78 °C overnight, the reaction was quenched with saturated aqueous NH4Cl, extracted with ethyl acetate, washed with brine, dried over anhydrous Na2SO4, concentrated, and purified by flash chromatography on silica gel (hexanes:ethyl acetate = 30:1) to give (R)-14 as a pale yellow oil (6.895 g, 76%). = −13.2 (c = 0.1, CHCl3).
(R)-6-(Benzyloxy)hex-4-yn-2-yl propionate ((R)-15). To a solution of (R)-14 (6.000 g, 29.37 mmol) in CH2Cl2 (150.0 mL) at 0 °C was added propionic acid (3.264 g, 44.06 mmol), DCC (9.091 g, 44.06 mmol), and DMAP (0.359 g, 2.94 mmol). The reaction mixture was stirred overnight, diluted with water, washed with saturated aqueous NaHCO3, extracted with CH2Cl2, washed with brine, dried over anhydrous Na2SO4, concentrated, and purified by flash chromatography on silica gel (hexanes:ethyl acetate = 50:1) to give (R)-15 as a pale yellow oil (7.647 g, 100%). = +33.8 (c = 0.1, CHCl3).
(R)-6-Hydroxyhexan-2-yl propionate ((R)-16). To a stirred solution of (R)-15 (4.100 g, 15.75 mmol) in MeOH (158.0 mL) was added Pd/C (10%, 1.300 g). The flask was evacuated and refilled with H2 (balloon). This process was repeated for 3 times. Upon stirring at room temperature for 24 h, the reaction mixture was filtered, concentrated, and purified by flash chromatography on silica gel (hexanes:ethyl acetate = 10:1) to give (R)-16 as a colorless oil (2.333 g, 85%). = −8.4 (c = 0.1, CHCl3).
(R)-6-Oxohexan-2-yl propionate ((R)-4). To a stirred solution of (R)-16 (1.900 g, 10.90 mmol) in CH2Cl2 (100.0 mL) was added silica gel (3.500 g) and PCC (3.400 g, 16.77 mmol). Upon stirring at room temperature for 12 h, the reaction mixture was precipitated by adding petroleum ether, filtered through a pad of Celite, concentrated, and purified by flash chromatography on silica gel (hexanes:ethyl acetate = 50:1) to give (R)-4 as a colorless oil (1.478 g, 79%). = −6.1 (c = 0.9, CHCl3).
General procedure for the preparation of 8-methyldec-6-en-2-yl propionate (1). To a stirred solution of sulfone 3 (1.0 equiv.) in THF (0.1 M) at −78 °C under Ar was added NaHMDS (2.0 M in THF, 1.2 equiv.). After 30 min, a solution of aldehyde 4 (1.2 equiv.) in THF (1.0 M) was added dropwise. The reaction mixture was slowly warmed to −50 °C and stirred overnight, quenched with saturated aqueous NH4Cl, extracted with ethyl acetate, washed with brine, dried over anhydrous Na2SO4, concentrated, and purified by flash chromatography on silica gel (hexanes:ethyl acetate = 100:1) to give 2 as a colorless oil.
To a stirred solution of 2 (1.0 equiv.) in EtOH (0.1 M) was added Pt/C (10%, 8.3% Pt). The flask was evacuated, and then filled with H2 (balloon). This process was repeated for 3 times. After 24 h, the reaction mixture was filtered, concentrated, and purified by flash chromatography on silica gel (hexanes:ethyl acetate = 100:1) to give 1 as a colorless oil.
(2S,8S)-8-Methyldecan-2-yl propionate ((2S,8S)-1). Sulfone (S)-3 (0.627 g, 2.33 mmol) and aldehyde (S)-4 (0.481 g, 2.79 mmol) were used to give: (2S,8S)-2 as a colorless oil (0.293 g, 56%), = +9.7 (c = 0.4, CHCl3), Z/E: 2/3 mixture, major: 1H-NMR (500 MHz, CDCl3) δ 5.35–5.22 (m, 1H), 5.15–5.08 (m, 1H), 4.95–4.86 (m, 1H), 2.36–2.24 (m, 3H), 2.07–1.92 (m, 2H), 1.62–1.22 (m, 6H), 1.20 (d, J = 6.3 Hz, 3H), 1.13 (t, J = 7.6 Hz, 3H), 0.91 (d, J = 6.7 Hz, 3H), 0.83 (t, J = 7.4 Hz, 3H); 13C-NMR (125 MHz, CDCl3) δ 174.15, 136.66, 127.86, 70.61, 35.55, 33.39, 30.21, 27.94, 27.18, 25.65, 21.00, 20.00, 11.91, 9.20; (2S,8S)-1 as a colorless oil (0.271 g, 92%), = +6.8 (c = 0.7, CHCl3), 1H-NMR (500 MHz, CDCl3) δ 4.92–4.86 (m, 1H), 2.29 (q, J = 7.6 Hz, 2H), 1.60–1.52 (m, 1H), 1.50–1.41 (m, 1H), 1.36–1.21 (m, 9H), 1.19 (d, J = 6.5 Hz, 3H), 1.13 (t, J = 7.6 Hz, 3H), 1.16–1.03 (m, 2H), 0.84 (t, J = 7.2 Hz, 3H), 0.83 (d, J = 6.3 Hz, 3H); 13C-NMR (125 MHz, CDCl3) δ 174.19, 70.81, 36.49, 35.96, 34.35, 29.78, 29.46, 27.95, 26.96, 25.43, 19.98, 19.18, 11.38, 9.22; HRMS (ESI) calcd for C14H28O2Na+ [M + Na]+: 251.1987, found: 251.1979.
(2R,8S)-8-Methyldecan-2-yl propionate ((2R,8S)-1). Sulfone (S)-3 (0.216 g, 0.80 mmol) and aldehyde (R)-4 (0.166 g, 0.96 mmol) were used to give: (2R,8S)-2 as a colorless oil (0.114 g, 63%), = +11.0 (c = 0.2, CHCl3), Z/E: 4/5 mixture, major: 1H-NMR (500 MHz, CDCl3) δ 5.35–5.21 (m, 2H), 4.95–4.86 (m, 1H), 2.34-2.25 (m, 3H), 2.07–1.90 (m, 2H), 1.61–1.23 (m, 6H), 1.19 (d, J = 6.3 Hz, 3H), 1.12 (t, J = 7.6 Hz, 3H), 0.91 (d, J = 6.7 Hz, 3H), 0.83 (t, J = 7.5 Hz, 3H); 13C-NMR (125 MHz, CDCl3) δ 174.66, 137.15, 128.36, 71.10, 36.03, 33.88, 30.71, 28.42, 27.66, 26.16, 21.51, 20.49, 12.44, 9.70; (2R,8S)-1 as a colorless oil (0.095 g, 83%), = +2.3 (c = 0.6, CHCl3), 1H-NMR (500 MHz, CDCl3) δ 4.92–4.86 (m, 1H), 2.28 (q, J = 7.6 Hz, 2H), 1.57–1.53 (m, 1H), 1.48–1.42 (m, 1H), 1.28–1.25 (m, 9H), 1.19 (d, J = 6.5 Hz, 3H), 1.12 (t, J = 7.6 Hz, 3H), 1.16–1.01 (m, 2H), 0.84 (t, J = 7.5 Hz, 3H), 0.83 (d, J = 6.0 Hz, 3H); 13C-NMR (125 MHz, CDCl3) δ 174.13, 70.79, 36.49, 35.96, 34.35, 29.78, 29.45, 27.94, 26.95, 25.42, 19.97, 19.17, 11.36, 9.20; HRMS (ESI) calcd for C14H28O2Na+ [M + Na]+: 251.1987, found: 251.1985.
(2S,8R)-8-Methyldecan-2-yl propionate ((2S,8R)-1). Sulfone (R)-3 (0.563 g, 2.09 mmol) and aldehyde (S)-4 (0.432 g, 2.51 mmol) were used to give: (2S,8R)-2 as a colorless oil (0.280 g, 59%), = −10.4 (c = 0.7, CHCl3), Z/E: 1/2 mixture, major: 1H-NMR (500 MHz, CDCl3) δ 5.36–5.06 (m, 2H), 4.96–4.84 (m, 1H), 2.35–2.23 (m, 3H), 2.09–1.89 (m, 2H), 1.65–1.21 (m, 6H), 1.19 (d, J = 6.2 Hz, 3H), 1.12 (t, J = 7.6 Hz, 3H), 0.91 (d, J = 6.7 Hz, 3H), 0.83 (t, J = 7.4 Hz, 3H); 13C-NMR (125 MHz, CDCl3) δ 174.14, 136.63, 127.85, 70.58, 35.52, 33.37, 30.20, 27.91, 27.14, 25.66, 21.01, 19.98, 11.93, 9.19; (2S,8R)-1 as a colorless oil (0.248 g, 88%), = −2.3 (c = 0.6, CHCl3), 1H-NMR (500 MHz, CDCl3) δ 4.94–4.84 (m, 1H), 2.28 (q, J = 7.6 Hz, 2H), 1.63–1.51 (m, 1H), 1.50–1.39 (m, 1H), 1.37–1.20 (m, 9H), 1.19 (d, J = 6.2 Hz, 3H), 1.12 (t, J = 7.6 Hz, 3H), 1.15–1.00 (m, 2H), 0.84 (t, J = 7.1 Hz, 3H), 0.83 (d, J = 5.3 Hz, 3H); 13C-NMR (125 MHz, CDCl3) δ 174.16, 70.79, 36.48, 35.95, 34.33, 29.78, 29.45, 27.93, 26.95, 25.43, 19.97, 19.17, 11.37, 9.21.
(2R,8R)-8-Methyldecan-2-yl propionate ((2R,8R)-1). Sulfone (R)-3 (0.453 g, 1.68 mmol) and aldehyde (R)-4 (0.346 g, 2.01 mmol) were used to give: (2R,8R)-2 as a colorless oil (0.236 g, 62%), = −9.1 (c = 0.6, CHCl3), Z/E: 2/3 mixture, major: 1H-NMR (500 MHz, CDCl3) δ 5.36–5.07 (m, 2H), 4.95–4.86 (m, 1H), 2.36–2.24 (m, 3H), 2.07–1.91 (m, 2H), 1.65–1.23 (m, 6H), 1.20 (d, J = 6.3 Hz, 3H), 1.13 (t, J = 7.6 Hz, 3H), 0.92 (d, J = 6.7 Hz, 3H), 0.83 (t, J = 7.4 Hz, 3H); 13C-NMR (125 MHz, CDCl3) δ 174.13, 136.66, 127.87, 70.60, 35.56, 33.39, 30.22, 27.94, 27.19, 25.66, 21.00, 20.00, 11.91, 9.20; (2R,8S)-1 as a colorless oil (0.186 g, 78%), = −8.6 (c = 0.3, CHCl3), 1H-NMR (500 MHz, CDCl3) δ 4.93–4.84 (m, 1H), 2.28 (q, J = 7.6 Hz, 2H), 1.62–1.51 (m, 1H), 1.50–1.39 (m, 1H), 1.36–1.20 (m, 9H), 1.19 (d, J = 6.3 Hz, 3H), 1.12 (t, J = 7.6 Hz, 3H), 1.15–1.02 (m, 2H), 0.84 (t, J = 7.4 Hz, 3H), 0.82 (d, J = 6.2 Hz, 3H); 13C-NMR (125 MHz, CDCl3) δ 174.16, 70.79, 36.48, 35.95, 34.33, 29.77, 29.44, 27.93, 26.95, 25.42, 19.97, 19.16, 11.36, 9.20.