Co2 and Co3 Mixed Cluster Secondary Building Unit Approach toward a Three-Dimensional Metal-Organic Framework with Permanent Porosity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Material Characterization of MOF 1
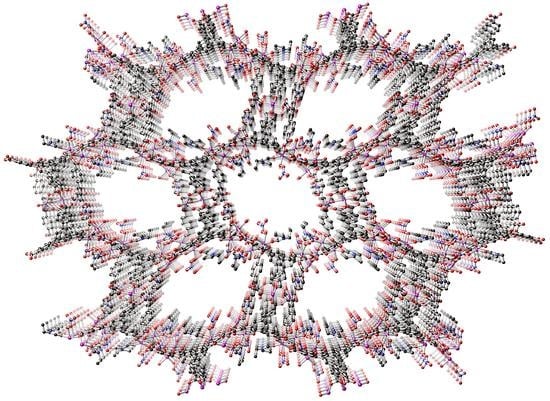
2.2. Crystal Structure Analysis of MOF 1
2.3. Gas Adsorption Behavior of MOF 1
3. Synthesis and Material Characterization
3.1. General
3.2. Synthesis of Co11(BTB)6(NO3)4(DEF)2(H2O)14 (MOF 1)
3.3. X-Ray Crystallography for MOF 1
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ikuno, T.; Zheng, J.; Vjunov, A.; Sanchez-Sanchez, M.; Ortuño, M.A.; Pahls, D.R.; Fulton, J.L.; Camaioni, D.M.; Li, Z.; Ray, D.; et al. Methane oxidation to methanol catalyzed by Cu-oxo clusters stabilized in NU-1000 metal-organic framework. J. Am. Chem. Soc. 2017, 139, 10294–10301. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-Z.; Wang, Z.U.; Wang, H.; Lu, J.; Yu, S.-H.; Jiang, H.-L. Singlet oxygen-engaged selective photo-oxidation over Pt nanocrystals/porphyrinic MOF: The roles of photothermal effect and Pt electronic state. J. Am. Chem. Soc. 2017, 139, 2035–2044. [Google Scholar] [CrossRef] [PubMed]
- Burgun, A.; Coghlan, C.J.; Huang, D.M.; Chen, W.; Horike, S.; Kitagawa, S.; Alvino, J.F.; Metha, G.F.; Sumby, C.J.; Doonan, C.J. Mapping-out catalytic processes in a metal-organic framework with single-crystal X-ray crystallography. Angew. Chem. Int. Ed. 2017, 56, 8412–8416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Manna, K.; Lin, W.B. Metal-organic frameworks stabilize solution-inaccessible cobalt catalysts for highly efficient broad-scope organic transformations. J. Am. Chem. Soc. 2016, 138, 3241–3249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wei, J.; Dong, J.; Liu, G.; Shi, L.; An, P.; Zhao, G.; Kong, J.; Wang, X.; Meng, X.; et al. Efficient visible-light-driven carbon dioxide reduction by a single-atom implanted metal-organic framework. Angew. Chem. Int. Ed. 2016, 55, 14310–14314. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Harris, T.D. Metal-organic frameworks as potential catalysts for industrial 1-butene production. ACS Cent. Sci. 2016, 2, 125–127. [Google Scholar] [CrossRef] [PubMed]
- Ikemoto, K.; Inokuma, Y.; Rissanen, K.; Fujita, M. X-ray snapshot observation of palladium-mediated aromatic bromination in a porous complex. J. Am. Chem. Soc. 2014, 136, 6892–6895. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Young, A.P.; Tsung, C.-K. Integration of biomolecules with metal-organic frameworks. Small 2017, 13, 1700880. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-X.; Yang, Y.-W. Metal-organic framework (MOF)-based drug/cargo delivery and cancer therapy. Adv. Mater. 2017, 1606134. [Google Scholar] [CrossRef] [PubMed]
- Teplensky, M.H.; Fantham, M.; Li, P.; Wang, T.C.; Mehta, J.P.; Young, L.J.; Moghadam, P.Z.; Hupp, J.T.; Farha, O.K.; Kaminski, C.F.; et al. Temperature treatment of highly porous zirconium-containing metal-organic frameworks extends drug delivery release. J. Am. Chem. Soc. 2017, 139, 7522–7532. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Xu, M.; Zheng, W.; Xu, T.; Deng, H.; Liu, J. Se/Ru-decorated porous metal-organic framework nanoparticles for the delivery of pooled siRNAs to reversing multidrug resistance in taxol-resistant breast cancer cells. ACS Appl. Mater. Interfaces 2017, 9, 6712–6724. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Zhang, Y.; Liu, L.; Wan, W.; Guo, P.; Nyström, A.M.; Zou, X. One-pot synthesis of metal-organic frameworks with encapsulated target molecules and their applications for controlled drug delivery. J. Am. Chem. Soc. 2016, 138, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Inokuma, Y.; Yoshioka, S.; Ariyoshi, J.; Arai, T.; Hitora, Y.; Takada, K.; Matsunaga, S.; Rissanen, K.; Fujita, M. X-ray analysis on the nanogram to microgram scale using porous complexes. Nature 2013, 495, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.; Fang, Y.; Joseph, E.; Wang, Q.; Li, J.; Banerjee, S.; Lollar, C.; Wang, X.; Zhou, H.-C. Enzyme-MOF (metal-organic framework) composites. Chem. Soc. Rev. 2017, 46, 3386–3401. [Google Scholar] [CrossRef] [PubMed]
- Lian, X.; Erazo-Oliveras, A.; Pellois, J.-P.; Zhou, H.-C. High efficiency and long-term intracellular activity of an enzymatic nanofactory based on metal-organic frameworks. Nat. Commun. 2017, 8, 2075. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Mao, C.; Luong, K.T.; Lin, Q.; Zhai, Q.-G.; Feng, P.; Bu, X. Framework cationization by preemptive coordination of open metal sites for anion-exchange encapsulation of nucleotides and coenzymes. Angew. Chem. Int. Ed. 2016, 55, 2768–2772. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Modica, J.A.; Howarth, A.J.; Vargas L, E.; Moghadam, P.Z.; Snurr, R.Q.; Mrksich, M.; Hupp, J.T.; Farha, O.K. Toward design rules for enzyme immobilization in hierarchical mesoporous metal-organic frameworks. Chem 2016, 1, 154–169. [Google Scholar] [CrossRef]
- Williams, D.E.; Dolgopolova, E.A.; Pellechia, P.J.; Palukoshka, A.; Wilson, T.J.; Tan, R.; Maier, J.M.; Greytak, A.B.; Smith, M.D.; Krause, J.A.; et al. Mimic of the green fluorescent protein β-barrel: Photophysics and dynamics of confined chromophores defined by a rigid porous scaffold. J. Am. Chem. Soc. 2015, 137, 2223–2226. [Google Scholar] [CrossRef] [PubMed]
- Towsif Abtab, S.M.; Alezi, D.; Bhatt, P.M.; Shkurenko, A.; Belmabkhout, Y.; Aggarwal, H.; Weseliński, Ł.J.; Alsadun, N.; Samin, U.; Hedhili, M.N.; et al. Reticular chemistry in action: A hydrolytically stable MOF capturing twice its weight in adsorbed water. Chem 2018, 4, 94–105. [Google Scholar] [CrossRef]
- Kim, H.; Yang, S.; Rao, S.R.; Narayanan, S.; Kapustin, E.A.; Furukawa, H.; Umans, A.S.; Yaghi, O.M.; Wang, E.N. Water harvesting from air with metal-organic frameworks powered by natural sunlight. Science 2017, 356, 430–434. [Google Scholar] [CrossRef] [PubMed]
- Cadiau, A.; Belmabkhout, Y.; Adil, K.; Bhatt, P.M.; Pillai, R.S.; Shkurenko, A.; Martineau-Corcos, C.; Maurin, G.; Eddaoudi, M. Hydrolytically stable fluorinated metal-organic frameworks for energy-efficient dehydration. Science 2017, 356, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Grunder, S.; Cordova, K.E.; Valente, C.; Furukawa, H.; Hmadeh, M.; Gandara, F.; Whalley, A.C.; Liu, Z.; Asahina, S.; et al. Large-pore apertures in a series of metal-organic frameworks. Science 2012, 336, 1018–1023. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S.; Li, Z.; Zheng, J.; Platero-Prats, A.E.; Mavrandonakis, A.; Pellizzeri, S.; Ferrandon, M.; Vjunov, A.; Gallington, L.C.; Webber, T.E.; et al. Sinter-resistant platinum catalyst supported by metal-organic framework. Angew. Chem. Int. Ed. 2018, 57, 909–913. [Google Scholar] [CrossRef] [PubMed]
- Zanon, A.; Verpoort, F. Metals@ZIFs: Catalytic applications and size selective catalysis. Coord. Chem. Rev. 2017, 353, 201–222. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, Q.; Jiang, H.-L. Metal-organic frameworks meet metal nanoparticles: Synergistic effect for enhanced catalysis. Chem. Soc. Rev. 2017, 46, 4774–4808. [Google Scholar] [CrossRef] [PubMed]
- Lismont, M.; Dreesen, L.; Wuttke, S. Metal-organic framework nanoparticles in photodynamic therapy: Current status and perspectives. Adv. Funct. Mater. 2017, 27, 1606314. [Google Scholar] [CrossRef]
- Fortea-Pérez, F.R.; Mon, M.; Ferrando-Soria, J.; Boronat, M.; Leyva-Pérez, A.; Corma, A.; Herrera, J.M.; Osadchii, D.; Gascon, J.; Armentano, D.; et al. The MOF-driven synthesis of supported palladium clusters with catalytic activity for carbene-mediated chemistry. Nat. Mater. 2017, 16, 760–766. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.-L.; Xu, Q. Immobilization of ultrafine metal nanoparticles to high-surface-area materials and their catalytic applications. Chem 2016, 1, 220–245. [Google Scholar] [CrossRef]
- Yang, Q.; Xu, Q.; Yu, S.-H.; Jiang, H.-L. Pd nanocubes@ZIF-8: Integration of plasmon-driven photothermal conversion with a metal-organic framework for efficient and selective catalysis. Angew. Chem. Int. Ed. 2016, 55, 3685–3689. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-S.; Ma, S.; Sun, D.; Parkin, S.; Zhou, H.-C. A mesoporous metal-organic framework with permanent porosity. J. Am. Chem. Soc. 2006, 128, 16474–16475. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, A.; Liu, L.; Tapperwijn, S.J.; Perl, D.; Coudert, F.-X.; Van Cleuvenbergen, S.; Verbiest, T.; van der Veen, M.A.; Telfer, S.G. Controlled partial interpenetration in metal-organic frameworks. Nat. Chem. 2016, 8, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.-L.; Makala, T.A.; Zhou, H.-C. Interpenetration control in metal-organic frameworks for functional applications. Coord. Chem. Rev. 2013, 257, 2232–2249. [Google Scholar] [CrossRef]
- Zhang, J.; Wojtas, L.; Larsen, R.W.; Eddaoudi, M.; Zaworotko, M.J. Temperature and concentration control over interpenetration in a metal-organic material. J. Am. Chem. Soc. 2009, 131, 17040–17041. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Ren, Z.G.; Deng, L.; Zhang, W.H.; Zhao, X.; Sun, Z.R.; Lang, J.P. Solvent effect-driven assembly of W/Cu/S cluster-based coordination polymers from the cluster precursor [Et4N][Tp*WS3(CuBr)3] and CuCN: Isolation, structures and enhanced NLO responses. Dalton Trans. 2015, 44, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.H.; Ren, Z.G.; Lang, J.P. Rational construction of functional molybdenum (tungsten)-copper-sulfur coordination oligomers and polymers from preformed cluster precursors. Chem. Soc. Rev. 2016, 45, 4995–5019. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, H.; Bhatt, P.M.; Bezuidenhout, C.X.; Barbour, L.J. Direct evidence for single-crystal to single-crystal switching of degree of interpenetration in a metal-organic framework. J. Am. Chem. Soc. 2014, 136, 3776–3779. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Friedrichs, O.; O’Keeffe, M. Three-periodic tilings and nets: Face-transitive tilings and edge-transitive nets revisited. Acta Crystallogr. Sect. A 2007, 63, 344–347. [Google Scholar] [CrossRef] [PubMed]
- Férey, G.; Mellot-Draznieks, C.; Serre, C.; Millange, F.; Dutour, J.; Surble, S.; Margiolaki, I. A chromium terephthalate-based solid with unusually large pore volumes and surface area. Science 2005, 309, 2040–2042. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Eddaoudi, M.; O’Keeffe, M.; Yaghi, O.M. Design and synthesis of an exceptionally stable and highly porous metal-organic framework. Nature 1999, 402, 276–279. [Google Scholar]
- Cavka, J.H.; Jakobsen, S.; Olsbye, U.; Guillou, N.; Lamberti, C.; Bordiga, S.; Lillerud, K.P. A new zirconium inorganic building brick forming metal organic frameworks with exceptional stability. J. Am. Chem. Soc. 2008, 130, 13850–13851. [Google Scholar] [CrossRef] [PubMed]
- Caskey, S.R.; Wong-Foy, A.G.; Matzger, A.J. Dramatic tuning of carbon dioxide uptake via metal substitution in a coordination polymer with cylindrical pores. J. Am. Chem. Soc. 2008, 130, 10870–10871. [Google Scholar] [CrossRef] [PubMed]
- Farha, O.K.; Eryazici, I.; Jeong, N.C.; Hauser, B.G.; Wilmer, C.E.; Sarjeant, A.A.; Snurr, R.Q.; Nguyen, S.T.; Yazaydın, A.Ö.; Hupp, J.T. Metal-organic framework materials with ultrahigh surface areas: Is the sky the limit? J. Am. Chem. Soc. 2012, 134, 15016–15021. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-R.; Timmons, D.J.; Zhou, H.-C. Interconversion between molecular polyhedra and metal-organic frameworks. J. Am. Chem. Soc. 2009, 131, 6368–6369. [Google Scholar] [CrossRef] [PubMed]
- Dutta, A.; Wong-Foy, A.G.; Matzger, A.J. Coordination copolymerization of three carboxylate linkers into a pillared layer framework. Chem. Sci. 2014, 5, 3729–3734. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Ding, N.N.; Zhang, W.H.; Chen, J.X.; Young, D.J.; Hor, T.S.A. Stitching 2D polymeric layers into flexible interpenetrated metal-organic frameworks within single crystals. Angew. Chem. Int. Ed. 2014, 53, 4628–4632. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zeng, C.; Luo, T.-Y.; Merg, A.D.; Jin, R.; Rosi, N.L. Establishing porosity gradients within metal-organic frameworks using partial postsynthetic ligand exchange. J. Am. Chem. Soc. 2016, 138, 12045–12048. [Google Scholar] [CrossRef] [PubMed]
- Deria, P.; Mondloch, J.E.; Karagiaridi, O.; Bury, W.; Hupp, J.T.; Farha, O.K. Beyond post-synthesis modification: Evolution of Metal-organic frameworks via building block replacement. Chem. Soc. Rev. 2014, 43, 5896–5912. [Google Scholar] [CrossRef] [PubMed]
- Karagiaridi, O.; Bury, W.; Mondloch, J.E.; Hupp, J.T.; Farha, O.K. Solvent-assisted linker exchange: An alternative to the de novo synthesis of unattainable metal-organic frameworks. Angew. Chem. Int. Ed. 2014, 53, 4530–4540. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Cahill, J.F.; Fei, H.; Prather, K.A.; Cohen, S.M. Postsynthetic ligand and cation exchange in robust metal-organic frameworks. J. Am. Chem. Soc. 2012, 134, 18082–18088. [Google Scholar] [CrossRef] [PubMed]
- Burnett, B.J.; Barron, P.M.; Hu, C.; Choe, W. Stepwise synthesis of metal-organic frameworks: Replacement of structural organic linkers. J. Am. Chem. Soc. 2011, 133, 9984–9987. [Google Scholar] [CrossRef] [PubMed]
- Al-Maythalony, B.A.; Alloush, A.M.; Faizan, M.; Dafallah, H.; Elgzoly, M.A.A.; Seliman, A.A.A.; Al-Ahmed, A.; Yamani, Z.H.; Habib, M.A.M.; Cordova, K.E.; et al. Tuning the interplay between selectivity and permeability of ZIF-7 mixed matrix membranes. ACS Appl. Mater. Interfaces 2017, 9, 33401–33407. [Google Scholar] [CrossRef] [PubMed]
- Karagiaridi, O.; Bury, W.; Sarjeant, A.A.; Stern, C.L.; Farha, O.K.; Hupp, J.T. Synthesis and characterization of isostructural cadmium zeolitic imidazolate frameworks via solvent-assisted linker exchange. Chem. Sci. 2012, 3, 3256–3260. [Google Scholar] [CrossRef]
- Liu, L.; Zhou, T.-Y.; Telfer, S.G. Modulating the performance of an asymmetric organocatalyst by tuning its spatial environment in a metal-organic framework. J. Am. Chem. Soc. 2017, 139, 13936–13943. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Telfer, S.G. Systematic ligand modulation enhances the moisture stability and gas sorption characteristics of quaternary metal-organic frameworks. J. Am. Chem. Soc. 2015, 137, 3901–3909. [Google Scholar] [CrossRef] [PubMed]
- Thorp-Greenwood, F.L.; Kulak, A.N.; Hardie, M.J. An infinite chainmail of M6L6 metallacycles featuring multiple borromean links. Nat. Chem. 2015, 7, 526–531. [Google Scholar] [CrossRef] [PubMed]
- Hirai, K.; Reboul, J.; Morone, N.; Heuser, J.E.; Furukawa, S.; Kitagawa, S. Diffusion-coupled molecular assembly: Structuring of coordination polymers across multiple length scales. J. Am. Chem. Soc. 2014, 136, 14966–14973. [Google Scholar] [CrossRef] [PubMed]
- Kimitsuka, Y.; Hosono, E.; Ueno, S.; Zhou, H.; Fujihara, S. Fabrication of porous cubic architecture of ZnO using Zn-terephthalate MOFs with characteristic microstructures. Inorg. Chem. 2013, 52, 14028–14033. [Google Scholar] [CrossRef] [PubMed]
- Koh, K.; Wong-Foy, A.G.; Matzger, A.J. A crystalline mesoporous coordination copolymer with high microporosity. Angew. Chem. Int. Ed. 2008, 47, 677–680. [Google Scholar] [CrossRef] [PubMed]
- Schoedel, A.; Boyette, W.; Wojtas, L.; Eddaoudi, M.; Zaworotko, M.J. A family of porous lonsdaleite-e networks obtained through pillaring of decorated kagomé lattice sheets. J. Am. Chem. Soc. 2013, 135, 14016–14019. [Google Scholar] [CrossRef] [PubMed]
- Tu, B.; Pang, Q.; Xu, H.; Li, X.; Wang, Y.; Ma, Z.; Weng, L.; Li, Q. Reversible redox activity in multicomponent metal-organic frameworks constructed from trinuclear copper pyrazolate building blocks. J. Am. Chem. Soc. 2017, 139, 7998–8007. [Google Scholar] [CrossRef] [PubMed]
- Xiong, G.; Yu, B.; Dong, J.; Shi, Y.; Zhao, B.; He, L.-N. Cluster-based MOFs with accelerated chemical conversion of CO2 through C-C bond formation. Chem. Commun. 2017, 53, 6013–6016. [Google Scholar] [CrossRef] [PubMed]
- Aizawa, S.-I.; Funahashi, S. Octahedral-tetrahedral equilibrium and solvent exchange of cobalt(II) ions in primary alkylamines. Inorg. Chem. 2002, 41, 4555–4559. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Ma, S.; Pechan, M.J.; Zhou, H.-C. Magnetic properties of a noninterpenetrating chiral porous cobalt metal-organic framewok. J. Appl. Phys. 2007, 101, 09E108. [Google Scholar] [CrossRef]
- Lee, Y.M.; Song, Y.J.; Poong, J.I.; Kim, S.H.; Koo, H.G.; Lee, J.A.; Kim, C.; Kim, S.-J.; Kim, Y. Novel chains constructed from heterotrinuclear units and 1,2-bis(4-pyridyl)ethane formulated as [M2M′(O2CPh)6](bpa) (M = Co, Zn, M′ = Co, Cd): Their catalytic activity. Inorg. Chem. Commun. 2010, 13, 101–104. [Google Scholar] [CrossRef]
- Fan, J.; Gan, L.; Kawaguchi, H.; Sun, W.Y.; Yu, K.B.; Tang, W.X. Reversible anion exchanges between the layered organic-inorganic hybridized architectures: Syntheses and structures of manganese(II) and copper(II) complexes containing novel tripodal ligands. Chem. Eur. J. 2003, 9, 3965–3973. [Google Scholar] [CrossRef] [PubMed]
- Mu, B.; Huang, Y.; Walton, K.S. A metal-organic framework with coordinatively unsaturated metal centers and microporous structure. CrystEngComm 2010, 12, 2347–2349. [Google Scholar] [CrossRef]
- Chin, J.M.; Chen, E.Y.; Menon, A.G.; Tan, H.Y.; Hor, A.T.S.; Schreyer, M.K.; Xu, J. Tuning the aspect ratio of NH2-MIL-53(Al) microneedles and nanorods via coordination modulation. CrystEngComm 2013, 15, 654–657. [Google Scholar] [CrossRef]
- Hafizovic, J.; Bjorgen, M.; Olsbye, U.; Dietzel, P.D.C.; Bordiga, S.; Prestipino, C.; Lamberti, C.; Lillerud, K.P. The inconsistency in adsorption properties and powder XRD data of MOF-5 is rationalized by framework interpenetration and the presence of organic and inorganic species in the nanocavities. J. Am. Chem. Soc. 2007, 129, 3612–3620. [Google Scholar] [CrossRef] [PubMed]
- Stamatatos, T.C.; Boudalis, A.K.; Pringouri, K.V.; Raptopoulou, C.P.; Terzis, A.; Wolowska, J.; McInnes, E.J.L.; Perlepes, S.P. Mixed-valence cobalt(II/III) carboxylate clusters: Co4IICo2III and CoIICo2III complexes from the use of 2-(hydroxymethyl)pyridine. Eur. J. Inorg. Chem. 2007, 5098–5104. [Google Scholar] [CrossRef]
- Chen, W.-X.; Zhuang, G.-L.; Zhao, H.-X.; Long, L.-S.; Huang, R.-B.; Zheng, L.-S. Magnetic and thermal properties of three ionothermally synthesized metal-carboxylate frameworks of [M3(ip)4][EMIm]2 (M = Co, Ni, Mn, H2ip = isophthalic acid, EMIm = 1-ethyl-3-methyl imidazolium). Dalton Trans. 2011, 40, 10237–10241. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Dong, W.-W.; Wu, Y.-P.; Wang, Y.-N.; Wang, C.; Li, D.-S.; Zhang, Q.-C. Two (3,6)-connected porous metal-organic frameworks based on linear trinuclear [Co3(COO)6] and paddlewheel dinuclear [Cu2(COO)4] SBUs: Gas adsorption, photocatalytic behaviour, and magnetic properties. J. Mater. Chem. A 2015, 3, 6962–6969. [Google Scholar] [CrossRef]
- Dolomanov, O.V.; Blake, A.J.; Champness, N.R.; Schröder, M. OLEX: New software for visualization and analysis of extended crystal structures. J. Appl. Cryst. 2003, 36, 1283–1284. [Google Scholar] [CrossRef]
- Wollmann, P.; Leistner, M.; Stoeck, U.; Gruenker, R.; Gedrich, K.; Klein, N.; Throl, O.; Graehlert, W.; Senkovska, I.; Dreisbach, F.; et al. High-throughput screening: Speeding up porous materials discovery. Chem. Commun. 2011, 47, 5151–5153. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Cryst. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Sumida, K.; Rogow, D.L.; Mason, J.A.; McDonald, T.M.; Bloch, E.D.; Herm, Z.R.; Bae, T.-H.; Long, J.R. Carbon dioxide capture in metal-organic frameworks. Chem. Rev. 2012, 112, 724–781. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Thallapally, P.K.; McGrail, B.P.; Brown, D.R.; Liu, J. Progress in adsorption-based CO2 capture by metal-organic frameworks. Chem. Soc. Rev. 2012, 41, 2308–2322. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-R.; Kuppler, R.J.; Zhou, H.-C. Selective gas adsorption and separation in metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1477–1504. [Google Scholar] [CrossRef] [PubMed]
- Feldblyum, J.I.; Liu, M.; Gidley, D.W.; Matzger, A.J. Reconciling the discrepancies between crystallographic porosity and guest access as exemplified by Zn-HKUST-1. J. Am. Chem. Soc. 2011, 133, 18257–18263. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wong-Foy, A.G.; Vallery, R.S.; William, E.; Frieze, J.; Schnobrich, K.; Gidley, D.W.; Matzger, A.J. Evolution of nanoscale pore structure in coordination polymers during thermal and chemical exposure revealed by positron annihilation. Adv. Mater. 2010, 22, 1598–1601. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-R.; Ma, Y.; McCarthy, M.C.; Sculley, J.; Yu, J.; Jeong, H.-K.; Balbuena, P.B.; Zhou, H.-C. Carbon dioxide capture-related gas adsorption and separation in metal-organic frameworks. Coord. Chem. Rev. 2011, 255, 1791–1823. [Google Scholar] [CrossRef]
- Armaghan, M.; Shang, X.J.; Yuan, Y.Q.; Young, D.J.; Zhang, W.H.; Hor, T.S.A.; Lang, J.P. Metal-organic frameworks via emissive metal-carboxylate zwitterion intermediates. ChemPlusChem 2015, 80, 1231–1234. [Google Scholar] [CrossRef]
- Chen, J.-X.; Zhao, H.-Q.; Li, H.-H.; Huang, S.-L.; Ding, N.-N.; Chen, W.-H.; Young, D.J.; Zhang, W.-H.; Andy Hor, T.S. Bent tritopic carboxylates for coordination networks: Clues to the origin of self-penetration. CrystEngComm 2014, 16, 7722–7730. [Google Scholar] [CrossRef]
- Chen, J.-X.; Chen, M.; Ding, N.-N.; Chen, W.-H.; Zhang, W.-H.; Hor, T.S.A.; Young, D.J. Transmetalation of a dodecahedral Na9 aggregate-based polymer: A facile route to water stable Cu(II) coordination networks. Inorg. Chem. 2014, 53, 7446–7454. [Google Scholar] [CrossRef] [PubMed]
- Sing, K.S.W.; Williams, R.T. The use of molecular probes for the characterization of nanoporous adsorbents. Part. Part. Syst. Charact. 2004, 21, 71–79. [Google Scholar] [CrossRef]
- Moellmer, J.; Celer, E.B.; Luebke, R.; Cairns, A.J.; Staudt, R.; Eddaoudi, M.; Thommes, M. Insights on adsorption characterization of metal-organic frameworks: A benchmark study on the novel soc-MOF. Microporous Mesoporous Mater. 2010, 129, 345–353. [Google Scholar] [CrossRef]
- Moon, H.R.; Kobayashi, N.; Suh, M.P. Porous metal-organic framework with coordinatively unsaturated MnII sites: Sorption properties for various gases. Inorg. Chem. 2006, 45, 8672–8676. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SADABS: Program for Empirical Absorption Correction of Area Detector Data; University of Göttingen: Göttingen, Germany, 1996. [Google Scholar]
- Sheldrick, G. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. Sect. C 2015, 71, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Sheng, D.; Xu, C.; Dai, X.; Silver, M.A.; Li, J.; Li, P.; Wang, Y.; Wang, Y.; Chen, L.; et al. Identifying the recognition site for selective trapping of 99TcO4− in a hydrolytically stable and radiation resistant cationic metal-organic framework. J. Am. Chem. Soc. 2017, 139, 14873–14876. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.V.; Manna, B.; Karmakar, A.; Sahu, A.; Ghosh, S.K. A water-stable cationic metal-organic framework as a dual adsorbent of oxoanion pollutants. Angew. Chem. Int. Ed. 2016, 55, 7811–7815. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds Co11(BTB)6(NO3)4(DEF)2(H2O)14 are available from the authors. |





| Parameter | Value |
|---|---|
| Molecular formula | C172H112Co11N6O64 |
| Formula weight | 3934.90 |
| Crystal system | Monoclinic |
| Space group | P21/n |
| a (Å) | 27.295(3) |
| b (Å) | 36.845(4) |
| c (Å) | 29.289(3) |
| β (°) | 108.940(2) |
| V (Å3) | 27,861(5) |
| Z | 2 |
| ρcalc (g cm−3) | 0.469 |
| F(000) | 3990 |
| µ (mm−1) | 0.347 |
| Total reflections | 483,464 |
| Unique reflections | 49,039 |
| Observed reflections | 21,553 |
| Rint | 0.1868 |
| Variables | 1138 |
| R1 a | 0.1225 |
| wR2 b | 0.2201 |
| GOF c | 1.130 |
| ρmax/ρmin(e Å−3) | 1.864/−2.042 |
| Co(1)–O(1)#1 | 2.048(6) | Co(1)–O(1) | 2.048(6) | Co(1)–O(7) | 2.077(5) |
| Co(1)–O(7)#1 | 2.077(5) | Co(1)–O(13) | 2.157(6) | Co(1)–O(13)#1 | 2.157(6) |
| Co(2)–O(2)#1 | 1.962(6) | Co(2)–O(8) | 1.984(5) | Co(2)–O(22) | 2.051(5) |
| Co(2)–O(14) | 2.122(5) | Co(2)–O(13) | 2.151(6) | Co(3)–O(12)#2 | 2.034(5) |
| Co(3)–O(17)#3 | 2.056(5) | Co(3)–O(30) | 2.082(7) | Co(3)–O(9) | 2.093(5) |
| Co(3)–O(26) | 2.104(6) | Co(3)–O(34) | 2.132(7) | Co(4)–O(11)#2 | 1.989(6) |
| Co(4)–O(18)#3 | 1.994(5) | Co(4)–O(19) | 2.102(7) | Co(4)–O(10) | 2.136(4) |
| Co(4)–O(9) | 2.186(4) | Co(4)–O(46) | 2.20(3) | Co(4)–O(20) | 2.228(7) |
| Co(5)–O(5)#4 | 2.007(5) | Co(5)–O(15) | 2.060(5) | Co(5)–O(3)#5 | 2.077(5) |
| Co(5)–O(44) | 2.082(6) | Co(5)–O(35) | 2.100(7) | Co(5)–O(43) | 2.195(8) |
| Co(6)–O(16) | 2.032(5) | Co(6)–O(6)#4 | 2.048(5) | Co(6)–O(45) | 2.059(6) |
| Co(6)–O(3)#5 | 2.137(5) | Co(6)–O(39) | 2.149(7) | Co(6)–O(4)#5 | 2.236(4) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chao, M.-Y.; Zhang, W.-H.; Lang, J.-P. Co2 and Co3 Mixed Cluster Secondary Building Unit Approach toward a Three-Dimensional Metal-Organic Framework with Permanent Porosity. Molecules 2018, 23, 755. https://doi.org/10.3390/molecules23040755
Chao M-Y, Zhang W-H, Lang J-P. Co2 and Co3 Mixed Cluster Secondary Building Unit Approach toward a Three-Dimensional Metal-Organic Framework with Permanent Porosity. Molecules. 2018; 23(4):755. https://doi.org/10.3390/molecules23040755
Chicago/Turabian StyleChao, Meng-Yao, Wen-Hua Zhang, and Jian-Ping Lang. 2018. "Co2 and Co3 Mixed Cluster Secondary Building Unit Approach toward a Three-Dimensional Metal-Organic Framework with Permanent Porosity" Molecules 23, no. 4: 755. https://doi.org/10.3390/molecules23040755
APA StyleChao, M. -Y., Zhang, W. -H., & Lang, J. -P. (2018). Co2 and Co3 Mixed Cluster Secondary Building Unit Approach toward a Three-Dimensional Metal-Organic Framework with Permanent Porosity. Molecules, 23(4), 755. https://doi.org/10.3390/molecules23040755