Tricycloalternarene Analogs from a Symbiotic Fungus Aspergillus sp. D and Their Antimicrobial and Cytotoxic Effects
Abstract
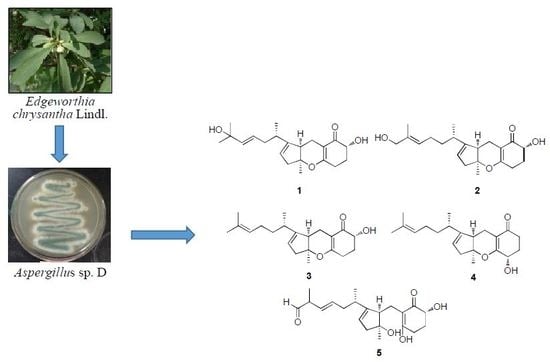
:1. Introduction
2. Results and Discussion
3. Experimental Section
3.1. General Experimental Procedures
3.2. Fungal Materials
3.3. Fermentation, Extraction, and Isolation
3.4. Biological Assays
3.4.1. Antimicrobial Test
3.4.2. Cytotoxicity Test
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Vadlapudi, V.; Borah, N.; Yellusani, K.R.; Gade, S.; Reddy, P.; Rajamanikyam, M.; Vempati, L.N.S.; Gubbala, S.P.; Chopra, P.; Upadhyayula, S.M.; et al. Aspergillus Secondary Metabolite Database, a resource to understand the secondary metabolome of Aspergillus genus. Sci. Rep. 2017, 7, 7325. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.W.; Tang, Y.F.; Ruan, C.F.; Bai, X.L. Bioactive secondary metabolites from the endophytic Aspergillus genus. Rec. Nat. Prod. 2016, 10, 1–16. [Google Scholar]
- Bai, Z.Q.; Lin, X.P.; Wang, J.F.; Liu, Y.H. New meroterpenoids from the endophytic fungus Aspergillus flavipes AIL8 derived from the mangrove plant Acanthus ilicifolius. Mar. Drugs 2015, 13, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, R.P.; Gunther, W.; Heinze, S.; Liebermann, B. New tricycloalternarenes produced by the phytopathogenic fungus Alternaria alternata. Phytochemistry 1999, 52, 593–599. [Google Scholar] [CrossRef]
- Yuan, L.; Zhao, P.J.; Ma, J.; Li, G.H.; Shen, Y.M. Tricycloalternarenes A–E: Five new mixed terpenoids from the endophytic fungal strain Alternaria alternata Ly83. Helv. Chim. Acta 2008, 91, 1588–1594. [Google Scholar] [CrossRef]
- Shi, X.; Wei, W.; Zhang, W.J.; Hua, C.P.; Chen, C.J.; Ge, H.M.; Tan, R.X.; Jiao, R.H. New tricycloalternarenes from fungus Alternaria sp. J. Asian Nat. Prod. Res. 2015, 17, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Mei, W.L.; Bo, Z.; Zhao, Y.X.; Zhong, H.M.; Chen, X.L.W.; Zeng, Y.B.; Dong, W.H.; Huang, J.L.; Proksch, P.; Dai, H.F. Meroterpenes from endophytic fungus A1 of mangrove plant Scyphiphora hydrophyllacea. Mar. Drugs 2012, 10, 1993–2001. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.X.; Bao, L.; Yang, X.L.; Guo, H.; Ren, B.; Guo, L.D.; Song, F.H.; Wang, W.Z.; Liu, H.W.; Zhang, L.X. Tricycloalternarenes F–H: Three new mixed terpenoids produced by an endolichenic fungus Ulocladium sp. using OSMAC method. Fitoterapia 2013, 85, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Kono, Y.; Gardner, J.M.; Suzuki, Y.; Takeuchi, S. Studies on host-selective toxins produced by a pathotype of Alternaria citri causing brown spot disease of mandarins. Agric. Biol. Chem. 1986, 50, 1597–1606. [Google Scholar] [CrossRef]
- Sun, H.; Gao, S.H.; Li, X.M.; Li, C.S.; Wang, B.G. Chemical constituents of marine mangrove-derived endophytic fungus Alternaria tenuissima EN-192. Chin. J. Oceanol. Limnol. 2013, 31, 464–470. [Google Scholar] [CrossRef]
- Sviridov, S.I.; Ermolinskii, B.S.; Belyakova, G.A.; Dzhavakhiya, V.G. Secondary metabolites of Ulocladium chartarum. Ulocladols A and B—New phytotoxins of terpenoid nature. Chem. Nat. Compd. 1991, 27, 566–571. [Google Scholar] [CrossRef]
- Liebermann, B.; Ellinger, R.; Günther, W.; Ihn, W.; Gallander, H. Tricycloalternarenes produced by Alternaria alternata related to ACTG-toxins. Phytochemistry 1997, 46, 297–303. [Google Scholar] [CrossRef]
- Yoiprommarat, S.; Srichomthong, K.; Deelai, S.; Suetrong, S.; Sakayaroj, J.; Bunyapaiboonsri, T.; Unagul, P. Secondary metabolites of the marine fungus Paradendryphiella arenariae BCC 17999. Bot. Mar. 2015, 27, 566–571. [Google Scholar] [CrossRef]
- Patton, T.; Barrett, J.; Brennan, J.; Moran, N. Use of a spectrophotometric bioassay for determination of microbial sensitivity to manuka honey. J. Microbiol. Methods 2006, 64, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Duan, Y.T.; Yao, Y.F.; Tang, D.J.; Thumar, N.; Teraiya, S.B.; Makawana, J.A.; Sang, T.L.; Wang, Z.C.; Tao, X.X.; Jiang, A.Q.; et al. Synthesis and biological evaluation of quinolineimidazole hybrids as potent telomerase inhibitors: A promising class of antitumor agents. RSC Adv. 2014, 4, 20382–20392. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds 1–5 are available from the authors. |



| Position | 1 | 2 | ||
|---|---|---|---|---|
| δH (J in Hz) | δC | δH (J in Hz) | δC | |
| 1 | 1.29 (3H, s) | 29.8 | 3.95 (2H, s) | 68.7 |
| 2 | 70.7 | 135.2 | ||
| 2′ | 1.29 (3H, s) | 29.8 | 1.62 (3H, s) | 23.2 |
| 3 | 5.59 (H, d, 15.6) | 139.6 | 5.25 (H, t, 7.6) | 125.4 |
| 4 | 5.50 (H, m) | 125.2 | 2.01 (2H, m) | 24.9 |
| 5 | 1.97 (H, m) 2.23 (H, m) | 27.8 | 1.50 (2H, m) | 34.6 |
| 6 | 2.08 (H, m) | 32.8 | 1.90 (H, m) | 31.1 |
| 6′ | 0.96, (3H, d, 6.9) | 19.4 | 0.96 (3H, d, 6.9) | 13.7 |
| 7 | 149.7 | 150.0 | ||
| 8 | 5.33 (H, s) | 120.4 | 5.34 (H, s) | 119.9 |
| 9 | 2.43 (H, m) 2.60 (H, m) | 44.9 | 2.36 (H, m) 2.60 (H, m) | 45.1 |
| 10 | 88.3 | 88.8 | ||
| 10′ | 1.43 (3H, s) | 23.4 | 1.45 (3H, s) | 20.2 |
| 11 | 2.75 (H, m) | 46.3 | 2.77 (H, m) | 46.5 |
| 12 | 2.49 (H, m) 2.73 (H, m) | 15.4 | 2.17 (H, m) 2.73 (H, m) | 14.9 |
| 13 | 105.2 | 105.1 | ||
| 14 | 171.8 | 172.9 | ||
| 15 | 2.34 (H, m) 2.49 (H, m) | 29.3 | 2.37 (m) 2.53 (m) | 27.8 |
| 16 | 1.77 (H, m) 2.08 (H, m) | 37.3 | 1.73 (H, m) 2.32 (H, m) | 29.4 |
| 17 | 4.04 (H, dd, 12.9, 5.4) | 71.6 | 4.02 (H, dd, 12.9, 5.4) | 71.0 |
| 18 | 197.8 | 197.8 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Zhao, Z.; Chen, J.; Bai, X.; Wang, H. Tricycloalternarene Analogs from a Symbiotic Fungus Aspergillus sp. D and Their Antimicrobial and Cytotoxic Effects. Molecules 2018, 23, 855. https://doi.org/10.3390/molecules23040855
Zhang H, Zhao Z, Chen J, Bai X, Wang H. Tricycloalternarene Analogs from a Symbiotic Fungus Aspergillus sp. D and Their Antimicrobial and Cytotoxic Effects. Molecules. 2018; 23(4):855. https://doi.org/10.3390/molecules23040855
Chicago/Turabian StyleZhang, Huawei, Ziping Zhao, Jianwei Chen, Xuelian Bai, and Hong Wang. 2018. "Tricycloalternarene Analogs from a Symbiotic Fungus Aspergillus sp. D and Their Antimicrobial and Cytotoxic Effects" Molecules 23, no. 4: 855. https://doi.org/10.3390/molecules23040855
APA StyleZhang, H., Zhao, Z., Chen, J., Bai, X., & Wang, H. (2018). Tricycloalternarene Analogs from a Symbiotic Fungus Aspergillus sp. D and Their Antimicrobial and Cytotoxic Effects. Molecules, 23(4), 855. https://doi.org/10.3390/molecules23040855