Furan- and Thiophene-2-Carbonyl Amino Acid Derivatives Activate Hypoxia-Inducible Factor via Inhibition of Factor Inhibiting Hypoxia-Inducible Factor-1
Abstract
:1. Introduction
2. Results and Discussion
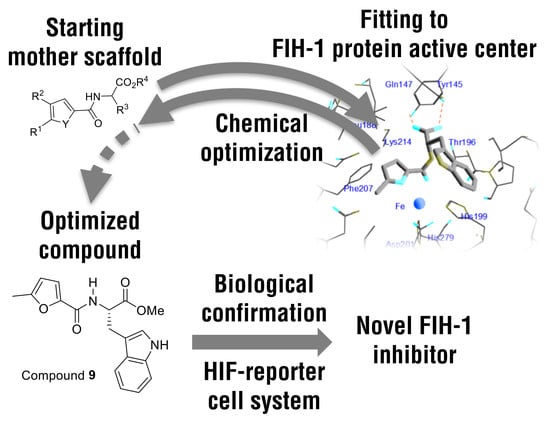
2.1. Design and Synthesis of New FIH-1 Inhibitors
2.2. Evaluation of HIF Activation by FIH-1 Inhibition
2.3. Evaluation of HIF Activation by Furan- and Thiophene-2-Carbonyl Amino Acid Derivatives under Hypoxic Conditions
2.4. Docking Simulations Using Furan- and Thiophene-2-Carbonyl Amino Acid Derivatives with FIH-1
2.5. Inactibation of HIF by FIH-1 Inhibitors under Normoxic Conditions
2.6. mRNA Expression in SK-N-BE(2) Cells
3. Conclusions
4. Materials and Methods
4.1. General Information
4.2. Preparation of 2-([1,1′-biphenyl]-4-yl)thiophene and 2-(4-phenoxyphenyl)thiophene [46]
4.3. Preparation of 2-((1,1′-biphenyl)-4-yl)thiophene-2-carboxylic acid, 2-(4-phenoxyphenyl)thiophene-2-carboxylic acid, 5-phenylthiophene-2-carboxylic acid, and 5-methylfuran-2-carboxylic acid [47]
4.4. Preparation of 5-phenylfuran-2-carboxylic acid
4.5. Preparation of 4-phenylthiophene-2-carboxylic acid
4.6. General Procedure for Furan- and Thiophene-2-Carbonyl Amino Acid Derivatives: Condensation Reaction of Furan- and Thiophene-2-Carboxylic Acids with Amino Acid Ester Hydrochlorides
4.7. Docking Simulation
4.8. Calculation of Solubility
4.9. Cell Culture
4.10. Evaluation of HIF Activity under Hypoxia Using a Luciferase Assay
4.11. MTS Assay
4.12. Evaluation of HIF Activity under Normoxia Using a Luciferase Assay
4.13. Silence of FIH-1 by Small Interfering RNA
4.14. Gene Expression Analysis
4.15. Statistical Analyses
Supplementary Materials
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ozer, A.; Bruick, R.K. Non-heme dioxygenases: Cellular sensors and regulators jelly rolled into one? Nat. Chem. Biol. 2007, 3, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Fraisl, P.; Aragones, J.; Carmeliet, P. Inhibition of oxygen sensors as a therapeutic strategy for ischaemic and inflammatory disease. Nat. Rev. Drug Discov. 2009, 8, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Rabinowitz, M.H. Inhibition of hypoxia-inducible factor prolyl hydroxylase domain oxygen sensors: Tricking the body into mounting orchestrated survival and repair responses. J. Med. Chem. 2013, 56, 9369–9402. [Google Scholar] [CrossRef] [PubMed]
- Miyata, T.; de Strihou, C.V. Diabetic nephropathy: A disorder of oxygen metabolism? Nat. Rev. Nephrol. 2010, 6, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Miyata, T.; Suzuki, N.; de Strihou, C.V. Diabetic nephropathy: Are there new and potentially promising therapies targeting oxygen biology? Kidney Int. 2013, 84, 693–702. [Google Scholar] [CrossRef] [PubMed]
- Ke, Q.; Costa, M. Hypoxia-inducible factor-1 (HIF-1). Mol. Pharmacol. 2006, 70, 1469–1480. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.C.; Holt-Martyn, J.P.; Schofield, C.J.; Ratcliffe, P.J. Pharmacological targeting of the HIF hydroxylases—A new field in medicine development. Mol. Asp. Med. 2016, 47–48, 54–75. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Wish, J.B. Hypoxia-inducible factor prolyl hydroxylase inhibitors: A potential new treatment for anemia in patients with CKD. Am. J. Kidney Dis. 2017, 69, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Lando, D.; Peet, D.J.; Whelan, D.A.; Gorman, J.J.; Whitelaw, M.L. Asparagine hydroxylation of the HIF transactivation domain a hypoxic switch. Science 2002, 295, 858–861. [Google Scholar] [CrossRef] [PubMed]
- Stolze, I.P.; Tian, Y.M.; Appelhoff, R.J.; Turley, H.; Wykoff, C.C.; Gleadle, J.M.; Ratcliffe, P.J. Genetic analysis of the role of the asparaginyl hydroxylase factor inhibiting hypoxia-inducible factor (HIF) in regulating HIF transcriptional target genes. J. Biol. Chem. 2004, 279, 42719–42725. [Google Scholar] [CrossRef] [PubMed]
- Hirota, K.; Semenza, G.L. Regulation of hypoxia-inducible factor 1 by prolyl and asparaginyl hydroxylases. Biochem. Biophys. Res. Commun. 2005, 338, 610–616. [Google Scholar] [CrossRef] [PubMed]
- Mahon, P.C.; Hirota, K.; Semenza, G.L. FIH-1: A novel protein that interacts with HIF-1α and VHL to mediate repression of HIF-1 transcriptional activity. Genes Dev. 2001, 15, 2675–2686. [Google Scholar] [CrossRef] [PubMed]
- Barrett, T.D.; Palomino, H.L.; Brondstetter, T.I.; Kanelakis, K.C.; Wu, X.; Haug, P.V.; Yan, W.; Young, A.; Hua, H.; Hart, J.C.; et al. Pharmacological characterization of 1-(5-chloro-6-(trifluoromethoxy)-1H-benzoimidazol-2-yl)-1H-pyrazole-4-carboxylic acid (JNJ-42041935), a potent and selective hypoxia-inducible factor prolyl hydroxylase inhibitor. Mol. Pharmacol. 2011, 79, 910–920. [Google Scholar] [CrossRef] [PubMed]
- Vachal, P.; Miao, S.; Pierce, J.M.; Guiadeen, D.; Colandrea, V.J.; Wyvratt, M.J.; Salowe, S.P.; Sonatore, L.M.; Milligan, J.A.; Hajdu, R.; et al. 1,3,8-Triazaspiro[4,5]decane-2,4-diones as efficacious pan-inhibitors of hypoxia-inducible factor prolyl hydroxylase 1–3 (HIF PHD1–3) for the treatment of anemia. J. Med. Chem. 2012, 55, 2945–2959. [Google Scholar] [CrossRef] [PubMed]
- Pergola, P.E.; Spinowitz, B.S.; Hartman, C.S.; Maroni, B.J.; Haase, V.H. Vadadustat, A novel oral HIF stabilizer, provides effective anemia treatment in nondialysis-dependent chronic kidney disease. Kidney Int. 2016, 90, 1115–1122. [Google Scholar] [CrossRef] [PubMed]
- Thevis, M.; Milosovich, S.; Licea-Perez, H.; Knecht, D.; Cavalier, T.; Schänzer, W. Mass spectrometric characterization of a prolyl hydroxylase inhibitor GSK1278863, its bishydroxylated metabolite, and its implementation into routine doping controls. Drug Test. Anal. 2016, 8, 858–863. [Google Scholar] [CrossRef] [PubMed]
- Eichner, D.; Van Wagoner, R.M.; Brenner, M.; Chou, J.; Leigh, S.; Wright, L.R.; Flippin, L.A.; Martinelli, M.; Krug, O.; Schänzer, W.; et al. Lmplementation of the prolyl hydroxylase inhibitor roxadustat (FG-4592) and its main metabolites into routine doping controls. Drug Test. Anal. 2017, 9, 1768–1778. [Google Scholar] [CrossRef] [PubMed]
- Mole, D.R.; Schlemminger, I.; McNeill, L.A.; Hewitson, K.S.; Pugh, C.W.; Ratcliffe, P.J.; Schofield, C.J. 2-Oxoglutarate analogue inhibitors of HIF prolyl hydroxylase. Bioorg. Med. Chem. Lett. 2003, 13, 2677–2680. [Google Scholar] [CrossRef]
- Mecinovic, J.; Loenarz, C.; Chowdhury, R.; Schofield, C.J. 2-Oxoglutarate analogue inhibitors of prolyl hydroxylase domain 2. Bioorg. Med. Chem. Lett. 2009, 19, 6192–6195. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.K.; Balan, C.; Allgeier, A.M.; Kasparian, A.; Viswanadhan, V.; Wilde, C.; Allen, J.R.; Yoder, S.C.; Biddlecome, G.; Hungate, R.W.; et al. Dipeptidyl-quinolone derivatives inhibit hypoxia inducible factor-1 alpha prolyl hydroxylases-1, -2, and -3 with altered selectivity. J. Comb. Chem. 2010, 12, 676–686. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.S.; Choi, Y.K.; Kim, J.W.; Park, Y.K.; Yang, E.G.; Ahn, D.R. Inhibition of a prolyl hydroxylase domain (PHD) by substrate analog peptides. Bioorg. Med. Chem. Lett. 2011, 21, 4325–4328. [Google Scholar] [CrossRef] [PubMed]
- Nangaku, M.; Izuhara, Y.; Takizawa, S.; Yamashita, T.; Fujii-Kuriyama, Y.; Ohneda, O.; Yamamoto, M.; de Strihou, C.V.; Hirayama, N.; Miyata, T. A novel class of prolyl hydroxylase inhibitors induces angiogenesis and exerts organ protection against ischemia. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 2548–2554. [Google Scholar] [CrossRef] [PubMed]
- Rosen, M.D.; Venkatesan, H.; Peltier, H.M.; Bembenek, S.D.; Kanelakis, K.C.; Zhao, L.X.; Leonard, B.E.; Hocutt, F.M.; Wu, X.D.; Palomino, H.L.; et al. Benzimidazole-2-pyrazole HIF prolyl 4-hydroxylase inhibitors as oral erythropoietin secretagogues. ACS Med. Chem. Lett. 2010, 1, 526–529. [Google Scholar] [CrossRef] [PubMed]
- Ivan, M.; Haberberger, T.; Gervasi, D.C.; Michelson, K.S.; Gunzler, V.; Kondo, K.; Yang, H.; Sorokina, I.; Conaway, R.C.; Conaway, J.W.; et al. Biochemical purification and pharmacological inhibition of a mammalian prolyl hydroxylase acting on hypoxia-inducible factor. Proc. Natl. Acad. Sci. USA 2002, 99, 13459–13464. [Google Scholar] [CrossRef] [PubMed]
- Warshakoon, N.C.; Wu, S.; Boyer, A.; Kawamoto, R.; Sheville, J.; Renock, S.; Xu, K.; Pokross, M.; Zhou, S.; Winter, C.; et al. Structure-based design, synthesis, and SAR evaluation of a new series of 8-hydroxyquinolines as HIF-1α prolyl hydroxylase inhibitors. Bioorg. Med. Chem. Lett. 2006, 16, 5517–5522. [Google Scholar] [CrossRef] [PubMed]
- Frohn, M.; Viswanadhan, V.; Pickrell, A.J.; Golden, J.E.; Muller, K.M.; Burli, R.W.; Biddlecome, G.; Yoder, S.C.; Rogers, N.; Dao, J.H.; et al. Structure-guided design of substituted aza-benzimidazoles as potent hypoxia inducible factor-1alpha prolyl hydroxylase-2 inhibitors. Bioorg. Med. Chem. Lett. 2008, 18, 5023–5026. [Google Scholar] [CrossRef] [PubMed]
- McDonough, M.A.; Li, V.; Flashman, E.; Chowdhury, R.; Mohr, C.; Lienard, B.M.; Zondlo, J.; Oldham, N.J.; Clifton, I.J.; Lewis, J.; et al. Cellular oxygen sensing: Crystal structure of hypoxia-inducible factor prolyl hydroxylase (PHD2). Proc. Natl. Acad. Sci. USA 2006, 103, 9814–9819. [Google Scholar] [CrossRef] [PubMed]
- Hong, Y.R.; Kim, H.T.; Lee, S.C.; Ro, S.; Cho, J.M.; Kim, I.S.; Jung, Y.H. [(4-Hydroxyl-benzo[4,5]thieno[3,2-c]pyridine-3-carbonyl)-amino]-acetic acid derivatives; HIF prolyl 4-hydroxylase inhibitors as oral erythropoietin secretagogues. Bioorg. Med. Chem. Lett. 2013, 23, 5953–5957. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Fu, Z.; Linke, S.; Chicher, J.; Gorman, J.J.; Visk, D.; Haddad, G.G.; Poellinger, L.; Peet, D.J.; Powell, F.; et al. The asparaginyl hydroxylase factor inhibiting HIF-1α is an essential regulator of metabolism. Cell Metab. 2010, 11, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Dayan, F.; Roux, D.; Brahimi-Horn, M.C.; Pouyssegur, J.; Mazure, N.M. The oxygen sensor factor-inhibiting hypoxia-inducible factor-1 controls expression of distinct genes through the bifunctional transcriptional character of hypoxia-inducible factor-1α. Cancer Res. 2006, 66, 3688–3698. [Google Scholar] [CrossRef] [PubMed]
- McDonough, M.A.; McNeill, L.A.; Tilliet, M.; Papamicaël, C.A.; Chen, Q.-Y.; Banerji, B.; Hewitson, K.S.; Schofield, C.J. Selective inhibition of factor inhibiting hypoxia-inducible factor. J. Am. Chem. Soc. 2005, 127, 7680–7681. [Google Scholar] [CrossRef] [PubMed]
- Banerji, B.; Conejo-Garcia, A.; McNeill, L.A.; McDonough, M.A.; Buck, M.R.G.; Hewitson, K.S.; Oldham, N.J.; Schofield, C.J. The inhibition of factor inhibiting hypoxia-inducible factor (FIH) by β-oxocarboxylic acids. Chem. Commun. 2005, 43, 5438–5440. [Google Scholar] [CrossRef] [PubMed]
- Conejo-Garcia, A.; McDonough, M.A.; Loenarz, C.; McNeill, L.A.; Hewitson, K.S.; Ge, W.; Liénard, B.M.; Schofield, C.J.; Clifton, I.J. Structural basis for binding of cyclic 2-oxoglutarate analogues to factor-inhibiting hypoxia-inducible factor. Bioorg. Med. Chem. Lett. 2010, 20, 6125–6128. [Google Scholar] [CrossRef] [PubMed]
- Chan, M.C.; Ilott, N.E.; Schödel, J.; Sims, D.; Tumber, A.; Lippl, K.; Mole, D.R.; Pugh, C.W.; Ratcliffe, P.J.; Ponting, C.P.; et al. Tuning the transcriptional response to hypoxia by inhibiting hypoxia-inducible factor (HIF) prolyl and asparaginyl hydroxylases. J. Biol. Chem. 2016, 291, 20661–20673. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.-M.; Yeoh, K.K.; Lee, M.K.; Eriksson, T.; Kessler, B.M.; Kramer, H.B.; Edelmann, M.J.; Willam, C.; Pugh, C.W.; Schofield, C.J.; et al. Differential sensitivity of hypoxia inducible factor hydroxylation sites to hypoxia and hydroxylase inhibitors. J. Biol. Chem. 2011, 286, 13041–13051. [Google Scholar] [CrossRef] [PubMed]
- Yeh, T.-L.; Leissing, T.; Abboud, M.I.; Thinnes, C.C.; Atasoylu, O.; Holt-Martyn, J.P.; Zhang, D.; Tumber, A.; Lippl, K.; Lohans, C.T.; et al. Molecular and cellular mechanisms of HIF prolyl hydroxylase inhibitors in clinical trials. Chem. Sci. 2017, 8, 7651–7668. [Google Scholar] [CrossRef] [PubMed]
- Dann, C.E.; Bruick, R.K.; Deisenhofer, J. Structure of factor-inhibiting hypoxia-inducible factor 1: An asparaginyl hydroxylase involved in the hypoxic response pathway. Proc. Natl. Acad. Sci. USA 2002, 99, 15351–15356. [Google Scholar] [CrossRef] [PubMed]
- Nagel, S.; Talbot, N.P.; Mecinovic, J.; Smith, T.G.; Buchan, A.M.; Schofield, C.J. Therapeutic manipulation of the HIF hydroxylases. Antioxid. Redox Signal. 2010, 12, 481–501. [Google Scholar] [CrossRef] [PubMed]
- Rose, N.R.; McDonough, M.A.; King, O.N.; Kawamura, A.; Schofield, C.J. Inhibition of 2-oxoglutarate dependent oxygenases. Chem. Soc. Rev. 2011, 40, 4364–4397. [Google Scholar] [CrossRef] [PubMed]
- Tsujita, T.; Kawaguchi, S.-I.; Dan, T.; Baird, L.; Miyata, T.; Yamamoto, M. Hypoxia-sensitive reporter system for high-throughput screening. Tohoku J. Exp. Med. 2015, 235, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Dayan, F.; Monticelli, M.; Pouyssegur, J.; Pecou, E. Gene regulation in response to graded hypoxia: The non-redundant roles of the oxygen sensors PHD and FIH in the HIF pathway. J. Theor. Biol. 2009, 259, 304–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guenzler-Pukall, V.W.Q.; Langsetmo, P.I.; Guo, G. Methods for Reducing Blood Pressure. WO Patent Application No. 2009058403A2009058401, 7 May 2009. [Google Scholar]
- Thomsen, R.; Christensen, M.H. Moldock: A new technique for high-accuracy molecular docking. J. Med. Chem. 2006, 49, 3315–3321. [Google Scholar] [CrossRef] [PubMed]
- Jiang, B.-H.; Zheng, J.Z.; Roe, L.R.; Semenza, G.L. Transactivation and inhibitory domains of hypoxia-inducible factor-1α. J. Biol. Chem. 1997, 272, 19253–19260. [Google Scholar] [CrossRef] [PubMed]
- Rose, N.R.; Ng, S.S.; Mecinovic, J.; Lienard, B.M.R.; Bello, S.H.; Sun, Z.; McDonough, M.A.; Oppermann, U.; Schofield, C.J. Inhibitor scaffolds for 2-oxoglutarate-dependent histone lysine demethylases. J. Med. Chem. 2008, 51, 7053–7056. [Google Scholar] [CrossRef] [PubMed]
- Amatore, C.; Jutand, A.; Negri, S.; Fauvarque, J.F. Efficient palladium-catalyzed synthesis of unsymmetrical donor-acceptor biaryls and polyaryls. J. Organomet. Chem. 1990, 390, 389–398. [Google Scholar] [CrossRef]
- Tserng, K.Y.; Bauer, L. Synthesis of 3-hydroxythienopyrimidine-2,4(1H,3H)-diones from 2,3-thiophenedicarboxylic and 3,4-thiophenedicarboxylic acids. J. Org. Chem. 1975, 40, 172–175. [Google Scholar] [CrossRef]
- Li, J.J.; Carson, K.G.; Trivedi, B.K.; Yue, W.S.; Ye, Q.; Glynn, R.A.; Miller, S.R.; Connor, D.T.; Roth, B.D.; Luly, J.R.; et al. Synthesis and structure–activity relationship of 2-amino-3-heteroaryl-quinoxalines as non-peptide, small-molecule antagonists for interleukin-8 receptor. Bioorg. Med. Chem. 2003, 11, 3777–3790. [Google Scholar] [CrossRef]
- Murasawa, S.; Iuchi, K.; Sato, S.; Noguchi-Yachide, T.; Sodeoka, M.; Yokomatsu, T.; Dodo, K.; Hashimoto, Y.; Aoyama, H. Small-molecular inhibitors of Ca2+-induced mitochondrial permeability transition (MPT) derived from muscle relaxant dantrolene. Bioorg. Med. Chem. 2012, 20, 6384–6393. [Google Scholar] [CrossRef] [PubMed]
- Itahara, T. Arylation of aromatic heterocycles with arenes and palladium(II) acetate. J. Org. Chem. 1985, 50, 5272–5275. [Google Scholar] [CrossRef]
- Frizler, M.; Schmitz, J.; Schulz-Fincke, A.-C.; Gütschow, M. Selective nitrile inhibitors to modulate the proteolytic synergism of cathepsins S and F. J. Med. Chem. 2012, 55, 5982–5986. [Google Scholar] [CrossRef] [PubMed]
- Horio, Y.; Ootake, Y.; Sawaki, S.; Inukai, S.; Agata, M.; Umezawa, M.; Goto, M. Tetrazoleacetic Acid Derivatives Having Aldose Reductase Inhibitory Activity. U.S. Patent Application No. 5252592A, 12 October 1993. [Google Scholar]
- Antonow, D.; Marrafa, T.; Dawood, I.; Ahmed, T.; Haque, M.R.; Thurston, D.E.; Zinzalla, G. Facile oxidation of electron-poor benzo[b]thiophenes to the corresponding sulfones with an aqueous solution of H2O2 and P2O5. Chem. Commun. 2010, 46, 2289–2291. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 1-44 are available from the authors. |













 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Entry | Y | R1 | R2 | R3 | R4 | R/S | Compound No. | 25% Activity a | 100 μM Activity b | Toxicity IC50 c | clogP |
| 1 | O | H | H | H | Me | - | 1 | 25 μM | 20% | >100 μM | 0.04 |
| 2 | S | H | H | H | Me | - | 2 | ND | 0% | >100 μM | 0.70 |
| 3 | O | H | H | -CH2-3-indolyl | Me | S | 3 | ND | 0% | >100 μM | 1.03 |
| 4 | O | H | H | -CH2-3-indolyl | Me | R | 4 | ND | 0% | >100 μM | 1.03 |
| 5 | S | H | H | -CH2-3-indolyl | Me | S | 5 | ND | 0% | >100 μM | 2.39 |
| 6 | S | H | H | -CH2-3-indolyl | Me | R | 6 | ND | 0% | >100 μM | 2.39 |
| 7 | O | Me | H | -CH2-Ph | Me | S | 7 | 25 μM | 22% | >100 μM | 1.82 |
| 8 | O | Me | H | -CH2-Ph | Me | R | 8 | ND | 2% | >100 μM | 1.82 |
| 9 | O | Me | H | -CH2-3-indolyl | Me | S | 9 | 25 μM | 22% | >100 μM | 1.37 |
| 10 | O | Me | H | -CH2-3-indolyl | Me | R | 10 | 6.3 μM | 0% | >100 μM | 1.37 |
| 11 | S | Me | H | H | H | - | 11 | 25 μM | 23% | >100 μM | 0.49 |
| 12 | S | Me | H | Me | H | S | 12 | ND | 18% | >100 μM | 1.25 |
| 13 | S | Me | H | Me | Et | S | 13 | ND | 0% | >100 μM | 1.85 |
| 14 | S | Me | H | -CH2-Ph | Me | R | 14 | ND | 0% | >100 μM | 3.19 |
| 15 | S | Me | H | -CH2-Ph | Et | S | 15 | 25 μM | 12% | >100 μM | 3.53 |
| 16 | S | Me | H | -CH2-3-indolyl | H | R | 16 | 100 μM | 30% | >100 μM | 2.47 |
| 17 | S | Me | H | -CH2-3-indolyl | Et | S | 17 | 100 μM | 49% | >100 μM | 3.07 |
| 18 | S | Me | H | -CH2-3-indolyl | Et | R | 18 | 100 μM | 52% | >100 μM | 3.07 |
| 19 | O | Ph | H | -CH2-Ph | H | S | 19 | ND | 0% | >100 μM | 2.96 |
| 20 | O | Ph | H | -CH2-Ph | Me | S | 20 | ND | 10% | >100 μM | 3.22 |
| 21 | S | Ph | H | Me | H | S | 21 | ND | 0% | >100 μM | 2.65 |
| 22 | S | Ph | H | Me | Et | S | 22 | ND | 16% | >100 μM | 3.25 |
| 23 | S | Ph | H | -CH2-Ph | H | S | 23 | ND | 0% | >100 μM | 4.32 |
| 24 | S | Ph | H | -CH2-Ph | Me | S | 24 | ND | 7% | >100 μM | 4.58 |
| 25 | S | Ph | H | -CH2-3-indolyl | Me | S | 25 | ND | 0% | >100 μM | 4.13 |
| 26 | S | H | Ph | H | Me | - | 26 | ND | 0% | >100 μM | 2.36 |
| 27 | S | H | Ph | -CH2-Ph | Et | S | 27 | ND | 2% | >100 μM | 4.87 |
| 28 | S | H | Ph | -CH2-3-indolyl | Me | S | 28 | 25 μM | 0% | >100 μM | 4.07 |
| 29 | S | H | Ph | -CH2-3-indolyl | Me | R | 29 | ND | 0% | >100 μM | 4.07 |
| 30 | S | H | Ph | -CH2-C6H4-4-OH | Me | S | 30 | 6.3 μM | 0% | 75 μM | 4.14 |
| 31 | S | 4-Ph-C6H4 | H | H | Me | - | 31 | ND | 0% | >100 μM | 4.09 |
| 32 | S | 4-Ph-C6H4 | H | -CH2-Ph | Et | S | 32 | ND | 0% | >100 μM | 6.60 |
| 33 | S | 4-PhO-C6H4 | H | H | Me | - | 33 | ND | 0% | >100 μM | 3.96 |
| 34 | S | 4-PhO-C6H4 | H | Me | Me | S | 34 | ND | 0% | >100 μM | 4.45 |
| 35 | S | 4-PhO-C6H4 | H | -CH2-Ph | Et | S | 35 | ND | 0% | >100 μM | 6.12 |
| 36 | S | 4-PhO-C6H4 | H | -CH2-3-indolyl | Me | S | 36 | ND | 0% | >100 μM | 5.66 |
 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Entry | Y | R3 | R4 | R/S | Compound No. | 25% Activity a | 100 μM Activity b | Toxicity IC50 c | clogP |
| 37 | O | -CH2-Ph | Et | S | 37 | ND | 0% | >100 μM | 2.88 |
| 38 | O | -CH2-3-indolyl | Me | S | 38 | 25 μM | 25% | >100 μM | 2.08 |
| 39 | S | H | Me | - | 39 | ND | 13% | >100 μM | 1.74 |
| 40 | S | Me | Et | S | 40 | ND | 0% | >100 μM | 2.57 |
| 41 | S | -CH2-Ph | Et | S | 41 | ND | 2% | >100 μM | 4.24 |
| 42 | S | -CH2-Ph | Me | R | 42 | ND | 0% | >100 μM | 3.91 |
| 43 | S | -CH2-3-indolyl | Me | S | 43 | ND | 0% | >100 μM | 3.45 |
| 44 | S | -CH2-C6H4-4-OH | Me | S | 44 | ND | 0% | >100 μM | 3.52 |
| Entry | Compound | Score (Plants) |
|---|---|---|
| 1 a | 7 (hydrolysate) | −63.8 |
| 2 a | 9 (hydrolysate) | −70.8 |
| 3 a | 10 (hydrolysate) | −57.7 |
| 4 | 16 | −29.9 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kawaguchi, S.-i.; Gonda, Y.; Yamamoto, T.; Sato, Y.; Shinohara, H.; Kobiki, Y.; Ichimura, A.; Dan, T.; Sonoda, M.; Miyata, T.; et al. Furan- and Thiophene-2-Carbonyl Amino Acid Derivatives Activate Hypoxia-Inducible Factor via Inhibition of Factor Inhibiting Hypoxia-Inducible Factor-1. Molecules 2018, 23, 885. https://doi.org/10.3390/molecules23040885
Kawaguchi S-i, Gonda Y, Yamamoto T, Sato Y, Shinohara H, Kobiki Y, Ichimura A, Dan T, Sonoda M, Miyata T, et al. Furan- and Thiophene-2-Carbonyl Amino Acid Derivatives Activate Hypoxia-Inducible Factor via Inhibition of Factor Inhibiting Hypoxia-Inducible Factor-1. Molecules. 2018; 23(4):885. https://doi.org/10.3390/molecules23040885
Chicago/Turabian StyleKawaguchi, Shin-ichi, Yuhei Gonda, Takuya Yamamoto, Yuki Sato, Hiroyuki Shinohara, Yohsuke Kobiki, Atsuhiko Ichimura, Takashi Dan, Motohiro Sonoda, Toshio Miyata, and et al. 2018. "Furan- and Thiophene-2-Carbonyl Amino Acid Derivatives Activate Hypoxia-Inducible Factor via Inhibition of Factor Inhibiting Hypoxia-Inducible Factor-1" Molecules 23, no. 4: 885. https://doi.org/10.3390/molecules23040885
APA StyleKawaguchi, S. -i., Gonda, Y., Yamamoto, T., Sato, Y., Shinohara, H., Kobiki, Y., Ichimura, A., Dan, T., Sonoda, M., Miyata, T., Ogawa, A., & Tsujita, T. (2018). Furan- and Thiophene-2-Carbonyl Amino Acid Derivatives Activate Hypoxia-Inducible Factor via Inhibition of Factor Inhibiting Hypoxia-Inducible Factor-1. Molecules, 23(4), 885. https://doi.org/10.3390/molecules23040885