Anti-Diabetic Effects of Acankoreagenin from the Leaves of Acanthopanax Gracilistylus Herb in RIN-m5F Cells via Suppression of NF-κB Activation
Abstract
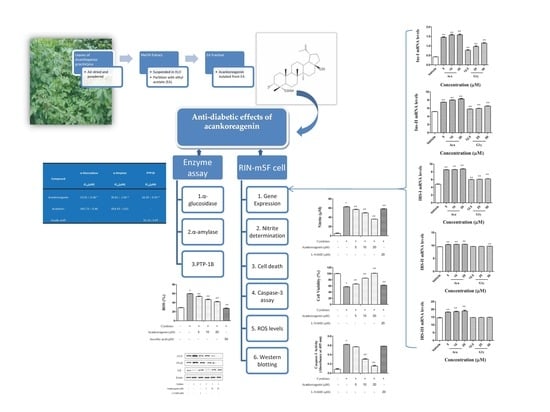
:1. Introduction
2. Results
2.1. Abilities of the Compound Acankoreagenin from LAG to Inhibit α-Glucosidase, α-Amylase, and PTP1B
2.2. Cell Viability
2.3. Effects of Acankoreagenin on GSIS in RIN-m5F Cells
2.4. Effects of Acankoreagenin on the Expression of Insulin Secretion-Related Gene in RIN-m5F Cells
2.5. Effects of Acankoreagenin on the Cytokine-Induced NO Production in RIN-m5F Cells
2.6. Effects of Acankoreagenin on the Cytokine-Induced Cell Death in RIN-m5F Cells
2.7. Effects of Acankoreagenin on the Cytokine-Induced Caspase-3 Activity in RIN-m5F Cells
2.8. Effects of Acankoreagenin on the Cytokine-Induced ROS Levels in RIN-m5F Cells
2.9. Effects of Acankoreagenin on the Cytokine-Induced Activation of NF-κB in RIN-m5F Cells
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Plant Materials and Isolation of Acankoreagenin from LAG
4.3. α-Glucosidase, α-Amylase, and PTP1B Inhibition Assay
4.4. Cell Cultures
4.5. MTT Assay for Cell Viability
4.6. Glucose-Stimulated Insulin Secretion Assay (GSIS)
4.7. qRT-PCR Analysis
4.8. Cytokine Treatment
4.9. Nitrite Determination and Prevention of Cytokine-Induced Cell Death
4.10. Caspase-3 Assay
4.11. Assay of Intracellular Reactive Oxygen Species (ROS) Levels
4.12. Western Blotting Analysis
4.13. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- World Health Organization. Global Report on Diabetes; World Health Organization: Geneva, Switzerland, 2016. [Google Scholar]
- Jung, N.; Um, J.; Dubon, M.J. Substance P preserves pancreatic β-cells in streptozotocin-induced type 1 diabetic mice. Biochem. Biophys. Res. Commun. 2017, 491, 958–965. [Google Scholar] [PubMed]
- Al-Hussaini, H.; Kilarkaje, N. Trans-resveratrol mitigates type 1 diabetes-induced oxidative DNA damage and accumulation of advanced glycation end products in glomeruli and tubules of rat kidneys. Toxicol. Appl. Pharm. 2018, 339, 97–109. [Google Scholar]
- Kumar, S.; Patial, V.; Soni, S.; Sharma, S.; Pratap, K.; Kumar, D.; Padwad, Y. Picrorhiza kurroa enhances β-Cell mass proliferation and insulin secretion in streptozotocin evoked β-Cell damage in rats. Front. Pharmacol. 2017, 8, 537–552. [Google Scholar] [PubMed]
- Ding, Y.; Zhang, Z.F.; Dai, X.Q.; Li, Y. Myricetin protects against cytokine-induced cell death in RIN-m5f β-cells. J. Med. Food 2012, 15, 733–740. [Google Scholar] [PubMed]
- Bae, U.J.; Jang, H.Y.; Lim, J.M.; Hua, L.; Ryu, J.H.; Park, B.H. Polyphenols isolated from Broussonetia kazinoki prevent cytokine-induced β-cell damage and the development of type 1 diabetes. Exp. Mol. Med. 2015, 47, e160–e169. [Google Scholar] [PubMed]
- Jeon, Y.D.; Kang, S.H.; Moon, K.H.; Lee, J.H.; Kim, D.G.; Kim, W.; Kim, J.S.; Ahn, B.Y.; Jin, J.S. The Effect of Aronia Berry on Type 1 Diabetes in vivo and in vitro. J. Med. Food 2018, 21, 244–253. [Google Scholar] [PubMed]
- Ju, S.M.; Youn, G.S.; Cho, Y.S.; Choi, S.Y.; Park, J. Celastrol ameliorates cytokine toxicity and pro-inflammatory immune responses by suppressing NF-κB activation in RINm5F beta cells. BMB Rep. 2015, 48, 172–177. [Google Scholar] [PubMed]
- Mandrup-Poulsen, T. Apoptotic signal transduction pathways in diabetes. Biochem. Pharmacol. 2003, 66, 1433–1440. [Google Scholar] [PubMed]
- Lai, X.; Kang, X.; Zeng, L.; Li, J.; Yang, Y.; Liu, D. The protective effects and genetic pathways of thorn grape seeds oil against high glucose-induced apoptosis in pancreatic β-cells. BMC Complement. Altern. Med. 2014, 14, 10. [Google Scholar]
- Lee, J.; Park, A.; Kim, M.J.; Lim, H.-J.; Rha, Y.-A.; Kang, H.-G. Spirulina Extract Enhanced a Protective Effect in Type 1 Diabetes by Anti-Apoptosis and Anti-ROS Production. Nutrients 2017, 9, 1363. [Google Scholar]
- Bae, U.J.; Lee, D.Y.; Song, M.Y.; Lee, S.M.; Park, J.W.; Ryu, J.H.; Park, B.H. A prenylated flavan from Broussonetia kazinoki prevents cytokine-induced β-cell death through suppression of nuclear factor-κB activity. Biol. Pharm. Bull. 2011, 34, 1026–1031. [Google Scholar] [PubMed]
- Rosim, M.P.; Nunes, V.; Lenzen, S.; Curi, R.; Azevedo-Martins, A.K. Culture Medium Fatty Acid Withdrawal Prompts Insulin Producing Cell Death. Endocrinol. Metab. Int. J. 2017, 5, 124–132. [Google Scholar]
- Ling, Z.; Heimberg, H.; Foriers, A.; Schuit, F.; Pipeleers, D. Differential expression of rat insulin I and II messenger ribonucleic acid after prolonged exposure of islet beta-cells to elevated glucose levels. Endocrinology 1998, 139, 491–495. [Google Scholar] [PubMed]
- Sesti, G.; Federici, M.; Hribal, M.L.; Lauro, D.; Sbraccia, P.; Lauro, R. Defects of the insulin receptor substrate (IRS) system in human metabolic disorders. FASEB J. 2001, 15, 2099–2111. [Google Scholar] [PubMed]
- Chávez-Silva, F.; Cerón-Romero, L.; Arias-Durán, L.; Navarrete-Vázquez, G.; Almanza-Pérez, J.; Román-Ramos, R.; Ramírez-Ávila, G.; Perea-Arango, I.; Villalobos-Molina, R.; Estrada-Soto, S. Antidiabetic effect of Achillea millefollium through multitarget interactions: α-glucosidases inhibition, insulin sensitization and insulin secretagogue activities. J. Ethnopharmacol. 2018, 212, 1–7. [Google Scholar] [PubMed]
- Xu, J.; Cao, J.; Yue, J.; Zhang, X.; Zhao, Y. New triterpenoids from acorns of Quercus liaotungensis and their inhibitory activity against α-glucosidase, α-amylase and protein-tyrosine phosphatase 1B. J. Funct. Foods 2018, 41, 232–239. [Google Scholar]
- Zhang, J.; Zhao, S.; Yin, P.; Yan, L.; Han, J.; Shi, L.; Zhou, X.; Liu, Y.; Ma, C. α-Glucosidase inhibitory activity of polyphenols from the burs of Castanea mollissima Blume. Molecules 2014, 19, 8373–8386. [Google Scholar] [PubMed]
- Jeong, S.Y.; Nguyen, P.H.; Zhao, B.T.; Ali, M.Y.; Choi, J.S.; Min, B.S.; Woo, M.H. Chemical constituents of Euonymus alatus (Thunb.) sieb. And their PTP1B and α-glucosidase inhibitory activities. Phytother. Res. 2015, 29, 1540–1548. [Google Scholar] [PubMed]
- Uddin, Z.; Song, Y.H.; Ullah, M.; Li, Z.; Kim, J.Y.; Park, K.H. Isolation and characterization of protein tyrosine phosphatase 1B (PTP1B) inhibitory polyphenolic compounds from Dodonaea viscosa and their kinetic analysis. Front. Chem. 2018, 6, 40–54. [Google Scholar] [PubMed]
- Kim, D.H.; Jung, H.A.; Sohn, H.S.; Kim, J.W.; Choi, J.S. Potential of icariin metabolites from Epimedium koreanum Nakai as antidiabetic therapeutic agents. Molecules 2017, 22, 986. [Google Scholar]
- Chinese Pharmacopoeia Commission. Chinese Pharmacopoeia of the People’s Republic of China; Medical Science and Technology Press: Beijing, China, 2015; Volume 1, p. 79. [Google Scholar]
- Wu, Z.Y.; Zhang, Y.B.; Zhu, K.K.; Luo, C.; Zhang, J.X.; Cheng, C.R.; Feng, R.H.; Yang, W.Z.; Zeng, F.; Wang, Y.; et al. Anti-inflammatory diterpenoids from the root bark of Acanthopanax gracilistylus. J. Nat. Prod. 2014, 77, 2342–2351. [Google Scholar] [PubMed]
- Zou, Q.P.; Liu, X.Q.; Lee, H.K.; Oh, O.J. Lupane-triterpenoids from the methanol extracts of leaves of Acanthopanax gracilistylus W. W. Smith. J. Lanzhou Univ. Nat. Sci. 2011, 47, 120–126. [Google Scholar]
- Li, X.J.; Zou, Q.P.; Wang, X.; Kim, K.W.; Lu, M.F.; Ko, S.K.; Yook, C.S.; Kim, Y.C.; Liu, X.Q. Lupane Triterpenes from Leave of Acanthopanax gracilistylus. Molecules 2018, 23, 87. [Google Scholar]
- Yook, C.S.; Liu, X.Q.; Chang, S.Y.; Park, S.Y.; Nohara, T. Lupane-triterpene glycosides from the leaves of Acanthopanax gracilistylus. Chem. Pharm. Bull. 2002, 50, 1383–1385. [Google Scholar] [PubMed]
- Liu, X.Q.; Chang, S.Y.; Park, S.Y.; Nohara, T.; Yook, C.S. A new lupane-triterpene glycoside from the leaves of Acanthopanax gracilistylus. Arch. Pharm. Res. 2002, 25, 831–836. [Google Scholar] [PubMed]
- Liu, X.Q.; Chang, S.Y.; Yook, C.S. Lupane-triterpenoids from the leaves of Acanthopanax gracilistylus. J. Lanzhou Univ. Nat. Sci. 2006, 42, 86–91. [Google Scholar]
- Liu, X.Q.; Zhang, C.Y.; Yin, W.J.; Liu, Z.X.; Yook, C.S. Analysis of volatile oil components of Acanthopanax gracilistylus. Chin. Tradit. Herb. Drugs 2001, 32, 1074–1075. [Google Scholar]
- Liu, X.Q.; Yook, C.S.; Chang, S.Y. Chemical constituents of Acanthopanax gracilistylus. Chin. Tradit. Herb. Drugs 2004, 35, 250–252. [Google Scholar]
- Zhang, J.Y.; Pu, S.B.; Qian, S.H.; Liu, D. New cerebrosides from Acanthopanax gracilistylus. Chin. J. Nat. Med. 2011, 9, 105–107. [Google Scholar]
- Liu, X.Q.; Chang, S.Y.; Ro, S.H.; Yook, C.S. Constituents of Acanthopanax gracilistylus W. W. Smith. Nat. Med. 2002, 56, 215–216. [Google Scholar]
- An, S.Y.; Qian, S.H.; Jiang, J.Q.; Wang, K.C. Chemical constituents in leaves of Acanthopanax gracilistylus. Chin. Tradit. Herb. Drugs 2009, 40, 1528–1534. [Google Scholar]
- Li, X.J.; Dai, L.; Li, Z.; Zhang, X.D.; Liu, X.Q.; Zou, Q.P.; Xie, X. Anti-inflammatory activities of lupane-triterpenoids in vitro and their phytochemical fingerprinting from leaves of Acanthopanax gracilistylus. Nat. Prod. Sci. 2015, 21, 104–110. [Google Scholar]
- Zou, Q.P.; Liu, X.Q.; Huang, J.J.; Yook, C.S.; Whang, W.K.; Lee, H.K.; Kwon, O.K. Inhibitory effects of lupane-type triterpenoid saponins from the leaves of Acanthopanax gracilistylus on lipopolysaccharide-induced TNF-α, IL-1β and high-mobility group box 1 release in macrophages. Mol. Med. Rep. 2017, 16, 9149–9156. [Google Scholar] [PubMed]
- Liu, X.Q.; Zou, Q.P.; Huang, J.J.; Yook, C.S.; Whang, W.K.; Lee, H.K.; Kwon, O.K. Inhibitory effects of 3α-hydroxy-lup-20(29)-en-23, 28-dioic acid on lipopolysaccharide-induced TNF-α, IL-1β, and the high mobility group box 1 release in macrophages. Biosci. Biotechnol. Biochem. 2017, 81, 1305–1313. [Google Scholar] [PubMed]
- Zhang, B.X.; Li, N.; Zhang, Z.P.; Liu, H.B.; Zhou, R.R.; Zhong, B.Y.; Zou, M.X.; Dai, X.H.; Xiao, M.F.; Liu, X.Q.; et al. Protective effect of Acanthopanax gracilistylus-extracted Acankoreanogenin A on mice with fulminant hepatitis. Int. Immunopharmacol. 2011, 11, 1018–1023. [Google Scholar] [PubMed]
- Ramírez-Rodríguez, A.M.; González-Ortiz, M.; Martínez-Abundis, E.; Acuña-Ortega, N. Effect of Ursolic Acid on Metabolic Syndrome, Insulin Sensitivity, and Inflammation. J. Med. Food 2017, 20, 882–886. [Google Scholar] [PubMed]
- Sandikapura, M.J.; Nyamathulla, S.; Noordin, M.I. Comparative antioxidant and antidiabetic effects of Syzygium polyanthumleaf and Momordica charantia fruit extracts. Pak. J. Pharm. Sci. 2018, 31, 623–635. [Google Scholar] [PubMed]
- Langer, O. Pharmacological treatment of gestational diabetes mellitus: Point/counterpoint. Am. J. Obstet. Gynecol. 2018, 28, 10–16. [Google Scholar]
- Darville, M.I.; Eizirik, D.L. Regulation by cytokines of the inducible nitric oxide synthase promoter in insulin-producing cells. Diabetologia 1998, 41, 1101–1108. [Google Scholar] [PubMed]
- Kim, E.K.; Kwon, K.B.; Song, M.Y.; Seo, S.W.; Park, S.J.; Ka, S.O.; Na, L.; Kim, K.A.; Ryu, D.G.; So, H.S.; et al. Genistein protects pancreatic β cells against cytokine-mediated toxicity. Mol. Cell. Endocrinol. 2007, 278, 18–28. [Google Scholar]
- Kim, E.K.; Kwon, K.B.; Song, M.Y.; Han, M.J.; Lee, J.H.; Lee, Y.R.; Lee, J.H.; Ryu, D.G.; Park, B.H.; Park, J.W. Flavonoids protect against cytokine-induced pancreatic β-cell damage through suppression of nuclear factor κB activation. Pancreas 2007, 35, E1–E9. [Google Scholar] [PubMed]
- Dai, L.; Liu, X.Q.; Xie, X.; Liu, H.Y. Characterization of stereostructure by X-ray and technology of extracting in combination hydrolysis in situ of acankoreanogenin from leaves of Acanthopanax gracilistylus W. W. Smith. J. Cent. South Univ. 2014, 21, 3063–3070. [Google Scholar]
- Geng, S.; Shan, S.; Ma, H.; Liu, B. Antioxidant activity and α-Glucosidase inhibitory activities of the polycondensate of catechin with glyoxylic acid. PLoS ONE 2016, 11, e0150412. [Google Scholar]
- Hemmati, M.; Serki, E.; Gholami, M.; Hoshyar, R. Effects of an ethanolic extract of Berberis vulgaris fruits on hyperglycemia and related gene expression in streptozotocin-induced diabetic rats. Clin. Phytosci. 2017, 2, 3. [Google Scholar]









| Compound | α-Glucosidase IC50 (μM) | α-Amylase IC50 (μM) | PTP1B IC50 (μM) |
|---|---|---|---|
| Acankoreagenin | 13.01 ± 0.38 * | 30.81 ± 1.04 * | 16.39 ± 0.54 * |
| Acarbose 1 | 661.73 ± 0.48 | 854.43 ± 0.81 | - |
| Ursolic acid 2 | - | - | 31.11 ± 0.47 |
| Primer | Forward/Reverse |
|---|---|
| β-actin | TCTGAACCCTAAGGCCAACCGTG |
| ATGGCATGAGGGAGCGCGTA | |
| Insulin I (INS I) | CAAACAGCACCTTTGTGGTCCTCAC |
| CACAATGCCACGCTTCTGCC | |
| Insulin II (INS II) | CAGCACCTTTGTGGTTCTCACTTGG |
| ATCCACGATGCCGCGCTTCT | |
| Insulin receptor substrate I (IRS-I) | AGAACGAGAAGAAGTGGCGGCAC |
| TGCAGCTGCAGAAGAGCCTG | |
| Insulin receptor substrate II (IRS-II) | AGCGAGAAGAAGTGGAAGAGCAAGG |
| TGACCAAGTCGGTGAGTGCG | |
| Insulin receptor substrate III (IRS-III) | CCATCTGAGGAAGCAGAAGTCCCA |
| TGACGATCAGGTGGCGCTGA |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, M.-X.; Yang, Y.; Zou, Q.-P.; Luo, J.; Zhang, B.-B.; Liu, X.-Q.; Hwang, E.-H. Anti-Diabetic Effects of Acankoreagenin from the Leaves of Acanthopanax Gracilistylus Herb in RIN-m5F Cells via Suppression of NF-κB Activation. Molecules 2018, 23, 958. https://doi.org/10.3390/molecules23040958
Lu M-X, Yang Y, Zou Q-P, Luo J, Zhang B-B, Liu X-Q, Hwang E-H. Anti-Diabetic Effects of Acankoreagenin from the Leaves of Acanthopanax Gracilistylus Herb in RIN-m5F Cells via Suppression of NF-κB Activation. Molecules. 2018; 23(4):958. https://doi.org/10.3390/molecules23040958
Chicago/Turabian StyleLu, Man-Xia, Yang Yang, Qin-Peng Zou, Jiao Luo, Bin-Bei Zhang, Xiang-Qian Liu, and Eun-Hee Hwang. 2018. "Anti-Diabetic Effects of Acankoreagenin from the Leaves of Acanthopanax Gracilistylus Herb in RIN-m5F Cells via Suppression of NF-κB Activation" Molecules 23, no. 4: 958. https://doi.org/10.3390/molecules23040958
APA StyleLu, M. -X., Yang, Y., Zou, Q. -P., Luo, J., Zhang, B. -B., Liu, X. -Q., & Hwang, E. -H. (2018). Anti-Diabetic Effects of Acankoreagenin from the Leaves of Acanthopanax Gracilistylus Herb in RIN-m5F Cells via Suppression of NF-κB Activation. Molecules, 23(4), 958. https://doi.org/10.3390/molecules23040958