Convenient Synthesis of Thiohydantoins, Imidazole-2-thiones and Imidazo[2,1-b]thiazol-4-iums from Polymer-Supported α-Acylamino Ketones
Abstract
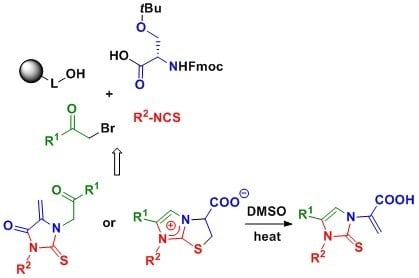
:1. Introduction
2. Results
3. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Fülöpová, V.; Soural, M. Mining the Chemical Space: Application of 2/4-Nitrobenzenesulfonamides in Solid-Phase Synthesis. ACS Comb. Sci. 2015, 17, 570–591. [Google Scholar] [CrossRef] [PubMed]
- Králová, P.; Fülöpová, V.; Maloň, M.; Volná, T.; Popa, I.; Soural, M. Stereoselective Polymer-Supported Synthesis of Morpholine- and Thiomorpholine-3-Carboxylic Acid Derivatives. ACS Comb. Sci. 2017, 19, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Pudelová, N.; Krchňák, V. Multiplicity of Diverse Heterocycles from Polymer-Supported α-Acylamino Ketones. J. Comb. Chem. 2009, 11, 851–859. [Google Scholar] [CrossRef] [PubMed]
- Fülöpová, V.; Krchňák, V. Solid-Phase Synthesis of Trisubstituted 2,5-Dihydrobenzo[f][1,2,5]thiadiazepine 1,1-Dioxide Derivatives. ACS Comb. Sci. 2014, 16, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Králová, P.; Maloň, M.; Volná, T.; Ručilová, V.; Soural, M. Polymer-Supported Stereoselective Synthesis of Benzoxazino[4,3-b][1,2,5]thiadiazepinone 6,6-Dioxides. ACS Comb. Sci. 2017, 19, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Králová, P.; Maloň, M.; Soural, M. Stereoselective Synthesis of Benzo[e][1,4]oxazino[4,3-a][1,4]diazepine-6,12-Diones with Two Diversity Positions. ACS Comb. Sci. 2017, 19, 770–774. [Google Scholar] [CrossRef] [PubMed]
- Ručilová, V.; Králová, P.; Soural, M. Synthesis of Disubstituted Pyrazino-Oxazine Derivatives with Controlled Stereochemistry. Eur. J. Org. Chem. 2017, 2017, 7034–7039. [Google Scholar] [CrossRef]
- Feldman, D.L. IBM PC Compatible Multi-Chip Module. U.S. Patent 5,742,844, 16 January 1998. [Google Scholar]
- Blanc, M.; Cussac, M.; Boucherle, A.; Leclerc, G. Synthesis and Immunomodulating Activity of 1-Amino-2-Thiohydantoin Derivatives. Eur. J. Med. Chem. 1992, 27, 839–843. [Google Scholar] [CrossRef]
- Janos, M.; Janos, E.; Sandor, H.; Tibor, T. Preparation and Fungicidal Activity of 5-Substituted Hydantoins and Their 2-Thio Analogs. J. Agric. Food. Chem. 1993, 41, 148–152. [Google Scholar]
- Sauli, M. Fungicidal Hydantoins Derivatives. U.S. Patent 3,823,240, 9 July 1974. [Google Scholar]
- Łażewska, D.; Maludziński, P.; Szymańska, E.; Kieć-Kononowicz, K. The Lipophilicity Estimation of 5-Arylidene Derivatives of (2-Thio)hydantoin with Antimycobacterial Activity. Biomed. Chromatogr. 2007, 21, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Santos, L.C.; Uchōa, F.T.; Canas, A.R.P.A.; Sousa, I.A.; Moura, R.O.; Lima, M.C.A.; Galdio, S.L.; Pitta, I.R.; Barbe, J. Synthesis and Anti-inflammatory Activity of New Thiazolidine-2,4-diones, 4-thioxothiazolidinones and 2-thioxoimidazolidinones. Heterocycl. Commun. 2005, 11, 121–128. [Google Scholar] [CrossRef]
- Knoefel, P.K.; Lehmann, G. The Anticonvulsant Action of Diphenyl Hydantoin and Some Related Compounds. J. Pharmacol. Exp. Ther. 1942, 76, 194–201. [Google Scholar]
- Habib, M.M.W.; Abdelfattah, M.A.O.; Abadi, A.H. Design and Synthesis of Novel Phenylpiperazine Derivatives as Potential Anticonvulsant Agents. Arch. Pharm. 2015, 348, 868–874. [Google Scholar] [CrossRef] [PubMed]
- Havera, H.J.; Goshen, G. 3-Substituted-5-Phenyl-5-Pyridyl Hydantoins. U.S. Patent 3,994,904, 30 November 1976. [Google Scholar]
- Tolman, R.L.; Gamsey, S.; Mehta, S.; Pongracz, K. Telomerase Inhibitors and Methods of Their Use. U.S. Patent 6,518,268, 11 February 2003. [Google Scholar]
- Rodgers, T.R.; LaMontagne, M.P.; Markovac, A.; Ash, A.B. Hydantoins as Antitumor Agents. J. Med. Chem. 1977, 20, 591–594. [Google Scholar] [CrossRef] [PubMed]
- Bell, C.; Conklin, G.L. Hydantoin Compounds and Methods of Preparation. U.S. Patent 3,452,041, 24 June 1969. [Google Scholar]
- Kodama, S. Thiocarbimide Reaction. J. Tokyo Chem. Soc. 1920, 41, 951–965. [Google Scholar]
- Reyes, S.; Burgess, K. On Formation of Thiohydantoins from Amino Acids under Acylation Conditions. J. Org. Chem. 2006, 71, 2507–2509. [Google Scholar] [CrossRef] [PubMed]
- Johnson, T.B.; Renfrew, A.G. Reseaches on Hydantoins. XLIII. Synthesis of the Polypeptide-Hydantoin: “Hydantoin-3-Acetic Acid”. J. Am. Chem. Soc. 1925, 47, 240–245. [Google Scholar] [CrossRef]
- Siddiqui, I.R.; Yadav, S.; Shamim, S.; Shireen; Waseem, M.A.; Srivastava, A.; Srivastava, A. Solid-Supported Cyclocondensation of Arylglyoxal on Ribosylthiourea under Microwave Activation: A Novel and Efficient Synthesis of N-Glycosylated-2-Thiohydantoins. Bull. Chem. Soc. Jpn. 2014, 87, 506–510. [Google Scholar] [CrossRef]
- Dadiboyena, S.; Nefzi, A. Parallel Solid-Phase Synthesis of Disubstituted 3-(1H-Benzo[d]imidazol-2-yl)imidazolidine-2,4-Diones and 3-(1H-Benzo[d]imidazol-2-yl)-2-Thioxoimidazolidin-4-Ones. Tetrahedron Lett. 2011, 52, 7030–7033. [Google Scholar] [CrossRef] [PubMed]
- Jullian, M.; Hernandez, A.; Maurras, A.; Puget, K.; Amblard, M.; Martinez, J.; Subra, G. N-Terminus FITC Labeling of Peptides on Solid Support: The Truth behind the Spacer. Tetrahedron Lett. 2009, 50, 260–263. [Google Scholar] [CrossRef]
- Lin, M.-J.; Sun, C.-M. Microwave-Assisted Traceless Synthesis of Thiohydantoin. Tetrahedron Lett. 2003, 44, 8739–8742. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, J.; Yao, Y.; Wang, C.; Zhang, J.; Shu, X.; Sun, X.; Li, Y.; Liu, K.; Yuan, H. Covalent binding design strategy: A prospective method for discovery of potent targeted anticancer agents. Eur. J. Med. Chem. 2017, 142, 493–505. [Google Scholar] [CrossRef] [PubMed]
- De Cesco, S.; Kurian, J.; Dufresne, C.; Mittermaier, A.K.; Moitessier, N. Covalent inhibitors design and discovery. Eur. J. Med. Chem. 2017, 138, 96–114. [Google Scholar] [CrossRef] [PubMed]
- Lagoutte, R.; Patouret, R.; Winssinger, N. Covalent inhibitors: An opportunity for rational target selectivity. Curr. Opinion Chem. Biol. 2017, 39, 54–63. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |







| Cmpd | R1 | R2 | Crude Purity (%) a | Final Purity (%) b | OverallVield (%) c | Ratio 8:9 At rt (%) c | Ratio 8:9 after Heating (%) c,d |
|---|---|---|---|---|---|---|---|
| 6{1,1} | Ph | H | 80 | 99 | 11 | - | - |
| 6{4,1} | 4-F-Ph | H | 69 | 98 | 11 | - | - |
| 6{5,1} | 4-Br-Ph | H | 75 | 97 | 7 | - | - |
| 6{6,1} | 4-NH2-3,5-diClPh | H | 68 | 98 | 8 | - | - |
| 6{7,1} | 3-thiophenyl | H | 85 | 97 | 8 | - | - |
| 7{1,1} | Ph | H | 85 | 99 | 19 | - | - |
| 7{6,1} | 4-Br-Ph | H | 71 | 98 | 16 | - | - |
| 7{7,1} | 3-thiophenyl | H | 72 | 99 | 15 | - | - |
| 8{1,2} | Ph | Bn | 80 | 98 | 53 | 93:7 | 0:100 |
| 8{1,3} | Ph | allyl | 90 | 98 | 34 | 100:0 | 8:92 |
| 8{1,4} | Ph | Et | 91 | 99 | 23 | 45:55 e | 11:89 |
| 8{2,2} | 4-Me-Ph | Bn | 96 | 99 | 38 | 97:3 | 3:97 |
| 8{2,5} | 4-Me-Ph | cyclohexylmethyl | 77 | 99 | 49 | 100:0 | NT |
| 8{3,3} | 4-MeO-Ph | allyl | 87 | 99 | 32 | 84:16 | 3:97 |
| 8{3,4} | 4-MeO-Ph | Et | 92 | 99 | 59 | 100:0 | 13:87 |
| 8{3,6} | 4-MeO-Ph | 2-(4-morpholino)ethyl | 95 | 99 | 39 | 60:40 e | 0:100 |
| 8{4,4} | 4-F-Ph | Et | 85 | 99 | 57 | 100:0 | 16:84 |
| 8{4,5} | 4-F-Ph | cyclohexylmethyl | 70 | 99 | 19 | 8:92 e | 8:92 |
| 8{5,2} | 4-Br-Ph | Bn | 91 | 98 | 28 | 98:2 | 9:91 |
| 8{5,3} | 4-Br-Ph | allyl | 69 | 99 | 18 | 100:0 | 29:71 |
| 8{5,6} | 4-Br-Ph | 2-(4-morpholino)ethyl | 90 | 98 | 17 | 88:12 | 0:100 |
| 8{7,2} | 3-thiophenyl | Bn | 97 | 98 | 26 | 97:3 | 0:100 |
| 8{7,3} | 3-thiophenyl | allyl | 83 | 98 | 24 | 89:11 | 0:100 |
| 8{7,6} | 3-thiophenyl | 2-(4-morpholino)ethyl | 95 | 98 | 39 | 82:18 | 82:18 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Králová, P.; Maloň, M.; Koshino, H.; Soural, M. Convenient Synthesis of Thiohydantoins, Imidazole-2-thiones and Imidazo[2,1-b]thiazol-4-iums from Polymer-Supported α-Acylamino Ketones. Molecules 2018, 23, 976. https://doi.org/10.3390/molecules23040976
Králová P, Maloň M, Koshino H, Soural M. Convenient Synthesis of Thiohydantoins, Imidazole-2-thiones and Imidazo[2,1-b]thiazol-4-iums from Polymer-Supported α-Acylamino Ketones. Molecules. 2018; 23(4):976. https://doi.org/10.3390/molecules23040976
Chicago/Turabian StyleKrálová, Petra, Michal Maloň, Hiroyuki Koshino, and Miroslav Soural. 2018. "Convenient Synthesis of Thiohydantoins, Imidazole-2-thiones and Imidazo[2,1-b]thiazol-4-iums from Polymer-Supported α-Acylamino Ketones" Molecules 23, no. 4: 976. https://doi.org/10.3390/molecules23040976
APA StyleKrálová, P., Maloň, M., Koshino, H., & Soural, M. (2018). Convenient Synthesis of Thiohydantoins, Imidazole-2-thiones and Imidazo[2,1-b]thiazol-4-iums from Polymer-Supported α-Acylamino Ketones. Molecules, 23(4), 976. https://doi.org/10.3390/molecules23040976