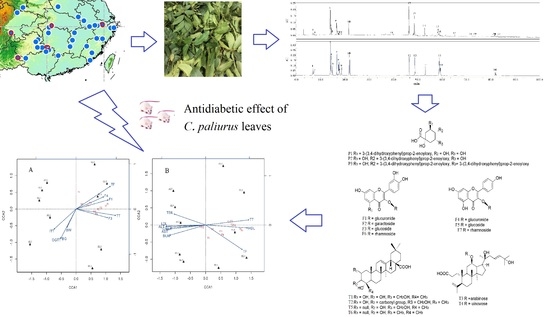
Antidiabetic Effect of Cyclocarya paliurus Leaves Depends on the Contents of Antihyperglycemic Flavonoids and Antihyperlipidemic Triterpenoids
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Compositions
2.2. Variation in Anti-Diabetic Properties
2.2.1. Variation in Body Weight, FBG, OGTT, and ITT
2.2.2. Variation in Lipid Levels
2.2.3. Variation in Liver and Kidney Function
3. Materials and Methods
3.1. Plant Materials
3.2. Chemical Reagents and References
3.3. Preparation of Ethanol and Aqueous Extracts
3.4. Determination of Chemical Compositions
3.5. Animals and Experimental Design
3.6. Body Weight and Fasting Blood Glucose Level (FBG)
3.7. Oral Glucose Tolerance Test (OGTT) and Insulin Tolerance Test (ITT)
3.8. Biochemical Analyses of the Serum Samples
3.9. Liver and Kidney Function
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Jia, W.; Gao, W.; Tang, L. Antidiabetic herbal drugs officially approved in China. Phytother. Res. 2003, 17, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Ahmad Aufa, Z.; Hassan, F.A.; Ismail, A.; Mohd Yusof, B.N.; Hamid, M. Chemical compositions and antioxidative and antidiabetic properties of underutilized vegetable palm hearts from Plectocomiopsis geminiflora and Eugeissona insignis. J. Agric. Food Chem. 2014, 62, 2077–2084. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.; Park, M.; Lee, H.C.; Kang, Y.H.; Kang, E.S.; Kim, S.K. Antidiabetic agents from medicinal plants. Curr. Med. Chem. 2006, 13, 1203–1218. [Google Scholar] [CrossRef] [PubMed]
- Dehghan-Kooshkghazi, M.; Mathers, J.C. Starch digestion, large-bowel fermentation and intestinal mucosal cell proliferation in rats treated with the α-glucosidase inhibitor acarbose. Br. J. Nutr. 2004, 91, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Umeno, A.; Horie, M.; Murotomi, K.; Nakajima, Y.; Yoshida, Y. Antioxidative and antidiabetic effects of natural polyphenols and isoflavones. Molecules 2016, 21, 708. [Google Scholar] [CrossRef] [PubMed]
- Phan, M.A.T.; Wang, J.; Tang, J.; Lee, Y.Z.; Ng, K. Evaluation of α-glucosidase inhibition potential of some flavonoids from Epimedium brevicornum. Food Sci. Technol. 2013, 53, 492–498. [Google Scholar] [CrossRef]
- Numonov, S.; Edirs, S.; Bobakulov, K.; Qureshi, M.N.; Bozorov, K.; Sharopov, F.; Aisa, H.A. Evaluation of the Antidiabetic Activity and Chemical Composition of Geranium collinum Root Extracts-Computational and Experimental Investigations. Molecules 2017, 22, 983. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Wang, J.; Wei, Z.; Zhu, Z. Methods to break seed dormancy in Cyclocarya paliurus (Batal) Iljinskaja. Sci. Hortic. 2006, 110, 305–309. [Google Scholar] [CrossRef]
- Liu, Y.; Fang, S.; Yang, W.; Shang, X.; Fu, X. Light quality affects flavonoid production and related gene expression in Cyclocarya paliurus. J. Photoch. Photobio. B. 2018, 179, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.H.; Dong, C.J.; Nie, S.P.; Li, F.; Wang, Z.J.; Shen, M.Y.; Xie, M.Y. Extraction, chemical composition and antioxidant activity of flavonoids from Cyclocarya paliurus (Batal.) Iljinskaja leaves. Food Chem. 2015, 186, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Kurihara, H.; Asami, S.; Shibata, H.; Fukami, H.; Tanaka, T. Hypolipemic effect of Cyclocarya paliurus (Batal) Iljinskaja in lipid-loaded mice. Biol. Pharm. Bull. 2003, 26, 383–385. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.H.; Shen, M.Y.; Xie, M.Y.; Nie, S.P.; Chen, Y.; Li, C.; Wang, Y.X. Ultrasonic-assisted extraction, antimicrobial and antioxidant activities of Cyclocarya paliurus (Batal.) Iljinskaja polysaccharides. Carbohyd. Polym. 2012, 89, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Qian, C.; Ding, S.; Shang, X.; Yang, W.; Fang, S. Effect of light regime and provenance on leaf characteristics, growth and flavonoid accumulation in Cyclocarya paliurus (Batal) Iljinskaja coppices. Bot. Stud. 2016, 57, 28. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Yang, W.; Chu, X.; Shang, X.; She, C.; Fu, X. Provenance and temporal variations in selected flavonoids in leaves of Cyclocarya paliurus. Food Chem. 2011, 124, 1382–1386. [Google Scholar] [CrossRef]
- Wright, M.; Byrd, J.; Gao, Y.; Stubblefield, J.; Park, H.; Dunlap, N. Isolation and structural clarification of triterpenes from Cyclocarya paliurus: Cyclocaric acid A and B. Planta Med. 2014, 80, PD19. [Google Scholar] [CrossRef]
- Kurihara, H.; Fukami, H.; Kusumoto, A.; Toyoda, Y.; Shibata, H.; Matsui, Y.; Tanaka, T. Hypoglycemic action of Cyclocarya paliurus (Batal.) Iljinskaja in normal and diabetic mice. Biosci. Biotechnol. Biochem. 2013, 67, 877–880. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, J.; Guan, X.L.; Li, J.; Deng, S.P.; Li, L.Q.; Yang, R.Y. Hypoglycemic effects and constituents of the barks of Cyclocarya paliurus and their inhibiting activities to glucosidase and glycogen phosphorylase. Fitoterapia 2011, 82, 1081–1085. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.W.; Ouyang, K.H.; Zhao, J.; Chen, H.; Xiong, L.; Wang, W.J. Structural characterization and hypolipidemic effect of Cyclocarya paliurus polysaccharide in rat. Int. J. Biol. Macromol. 2016, 91, 1073–1080. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.B.; Zhao, J.; Chen, H.; Xiong, L.; Wang, W.J. Polysaccharides from Cyclocarya paliurus: Chemical composition and lipid-lowering effect on rats challenged with high-fat diet. J. Funct. Foods 2017, 36, 262–273. [Google Scholar] [CrossRef]
- Wang, Q.; Jiang, C.; Fang, S.; Wang, J.; Ji, Y.; Shang, X.; Zhang, J. Antihyperglycemic, antihyperlipidemic and antioxidant effects of ethanol and aqueous extracts of Cyclocarya paliurus leaves in type 2 diabetic rats. J. Ethnopharmacol. 2013, 150, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Wang, Q.; Wei, Y.; Yao, N.; Wu, Z.; Ma, Y.; Zhang, J. Cholesterol-lowering effects and potential mechanisms of different polar extracts from Cyclocarya paliurus leave in hyperlipidemic mice. J. Ethnopharmacol. 2015, 176, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Jiang, C.; Yao, N.; Li, Y.; Wang, Q.; Fang, S.; Zhang, J. Antihyperlipidemic effect of Cyclocarya paliurus (Batal.) Iljinskaja extract and inhibition of apolipoprotein B48 overproduction in hyperlipidemic mice. J. Ethnopharmacol. 2015, 166, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.F.; Meng, F.C.; Cao, L.J.; Jiang, C.H.; Zhao, M.G.; Shang, X.L.; Yin, Z.Q. Triterpenoids from Cyclocarya paliurus and their inhibitory effect on the secretion of apoliprotein B48 in Caco-2 cells. Phytochemistry 2017, 142, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Fang, S.; Yin, Z.; Fu, X.; Shang, X.; Yang, W.; Yang, H. Chemical Fingerprint and Multicomponent Quantitative Analysis for the Quality Evaluation of Cyclocarya paliurus Leaves by HPLC-Q-TOF-MS. Molecules 2017, 22, 1927. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wen, K.S.; Ruan, X.; Zhao, Y.X.; Wei, F.; Wang, Q. Response of Plant Secondary Metabolites to Environmental Factors. Molecules 2018, 23, 762. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Hogan, S.; Li, J.; Sun, S.; Canning, C.; Zheng, S.J.; Zhou, K. Grape skin extract inhibits mammalian intestinal α-glucosidase activity and suppresses postprandial glycemic response in streptozocin-treated mice. Food Chem. 2011, 126, 466–471. [Google Scholar] [CrossRef]
- Shehata, A.M.; Quintanilla-Fend, L.; Bettio, S.; Singh, C.B.; Ammon, H.P.T. Prevention of multiple low-dose streptozotocin (MLD-STZ) diabetes in mice by an extract from gum resin of Boswellia serrata (BE). Phytomedicine 2011, 18, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Du, N.; Wang, L.; Wang, X.; Xiao, Y.; Zhang, K.; Wang, P. Conjugated fatty acid-rich oil from Gynostrmma pentaphyllum seed can ameliorate lipid and glucose metabolism in type 2 diabetes mellitus mice. Food Funct. 2017, 8, 3696–3706. [Google Scholar] [CrossRef] [PubMed]
- Mensah-Brown, E.P.K.; Al Rabesi, Z.; Shahin, A.; Al Shamsi, M.; Arsenijevic, N.; Hsu, D.K.; Lukic, M.L. Targeted disruption of the galectin-3 gene results in decreased susceptibility to multiple low dose streptozotocin-induced diabetes in mice. Clin. Immunol. 2009, 130, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.K.; Gao, J.; Zhu, D.N. Kaempferol and quercetin isolated from Euonymus alatus improve glucose uptake of 3T3-L1 cells without adipogenesis activity. Life Sci. 2008, 82, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, P.; Prajapati, A.K. Quercetin in anti-diabetic research and strategies for improved quercetin bioavailability using polymer-based carriers-a review. RSC Advances 2015, 5, 97547–97562. [Google Scholar] [CrossRef]
- Yao, Y.; Chen, F.; Wang, M.; Wang, J.; Ren, G. Antidiabetic activity of Mung bean extracts in diabetic KK-Ay mice. J. Agric. Food Chem. 2008, 56, 8869–8873. [Google Scholar] [CrossRef] [PubMed]
- Somova, L.O.; Nadar, A.; Rammanan, P.; Shode, F.O. Cardiovascular, antihyperlipidemic and antioxidant effects of oleanolic and ursolic acids in experimental hypertension. Phytomedicine 2003, 10, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Ejelonu, O.C.; Elekofehinti, O.O.; Adanlawo, I.G. Tithonia diversifolia saponin-blood lipid interaction and its influence on immune system of normal wistar rats. Biomed. Phar. 2017, 87, 589–595. [Google Scholar] [CrossRef] [PubMed]
- James, D.B.; Elebo, N.; Sanusi, A.M.; Odoemene, L. Some biochemical effect of intraperitoneal administration of Phyllanthus amarus aquoeus extacts on normaglycemic albino rats. Asian J. Med. Sci. 2010, 2, 7–10. [Google Scholar]
- Kew, M.C. Serum aminotransferase concentration as evidence of hepatocellular damage. Lancet 2000, 355, 591–592. [Google Scholar] [CrossRef]
- Ostfeld, R.; Spinelli, M.; Mookherjee, D.; Holtzman, D.; Shoyeb, A.; Schaefer, M.; Duc, Y. The association of blood urea nitrogen levels and coronary artery disease. Einstein J. Biol. Med. 2016, 25, 3–7. [Google Scholar] [CrossRef]
- Cao, J.; Wang, S.; Yao, C.; Xu, Z.; Xu, X. Hypolipidemic effect of porphyran extracted from Pyropia yezoensis in ICR mice with high fatty diet. J. Appl. Phycol. 2016, 28, 1315–1322. [Google Scholar] [CrossRef]
- Shi, F.; Li, J.; Yang, L.; Hou, G.; Ye, M. Hypolipidemic effect and protection ability of liver-kidney functions of melanin from Lachnum YM226 in high-fat diet fed mice. Food Funct. 2018, 9, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Wang, X.; Wang, H.; Shen, B.; He, X.; Gu, W.; Wu, Q. Extraction optimization, isolation, preliminary structural characterization and antioxidant activities of the cell wall polysaccharides in the petioles and pedicels of Chinese herbal medicine Qian (Euryale ferox Salisb.). Int. J. Biol. Macromol. 2014, 64, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, K.; Viswanad, B.; Asrat, L.; Kaul, C.L.; Ramarao, P. Combination of high-fat diet-fed and low-dose streptozotocin-treated rat: a model for type 2 diabetes and pharmacological screening. Pharmacol. Res. 2005, 52, 313–320. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds 4-O-caffeoylquinic acid, 3-O-caffeoylquinic acid, quercetin-3-O-glucuronide, isoquercitrin, quercetin-3-O-galactoside, kaempferol-3-O-glucuronide, kaempferol- 3-O-glucoside, kaempferol-3-O-rhamnoside, quercetin-3-O-rhamnoside, 4, 5-di-O-caffeoylquinic acid, arjunolic acid, cyclocaric acid B, pterocaryoside A, pterocaryoside B, hederagenin and oleanolic acid are available from the authors. |






| Compounds | LOD (ng/mL) | LOQ (ng/mL) | EL1 | EL2 | EL3 | EL4 | EL5 | AL1 | AL2 | AL3 | AL4 | AL5 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 3-O-caffeoylquinic acid (P1) | 64.25 | 214.21 | 0.35 ± 0.011 cd | 1.38 ± 0.153 a | 0.39 ± 0.003 cd | 0.45 ± 0.028 bc | 0.31 ± 0.003 d | 0.09 ± 0.003 e | 1.30 ± 0.028 a | 0.55 ± 0.006 b | 0.04 ± 0.003 e | 0.09 ± 0.006 e |
| 4-O-caffeoylquinic acid (P2) | 57.93 | 197.58 | 0.03 ± 0.001 h | 0.08 ± 0.003 e | 0.28 ± 0.003 b | 0.11 ± 0.003 d | 0.08 ± 0.002 e | 0.04 ± 0.001 g | 0.23 ± 0.003 c | 0.35 ± 0.003 a | 0.01 ± 0.001 i | 0.05 ± 0.003 f |
| 4,5-di-O-caffeoylquinic acid (P3) | 58.97 | 201.22 | 0.05 ± 0.003 d | 0.10 ± 0.001 c | 0.42 ± 0.028 a | 0.16 ± 0.003 b | 0.08 ± 0.003 cd | nd | nd | nd | nd | nd |
| quercetin-3-O-glucuronide (F1) | 40.28 | 128.74 | 2.15 ± 0.010 b | 1.57 ± 0.006 d | 2.12 ± 0.003 c | 2.38 ± 0.005 a | 0.78 ± 0.003 g | 0.33 ± 0.003 h | 1.25 ± 0.028 e | 0.98 ± 0.003 f | 0.30 ± 0.009 i | 0.34 ± 0.009 h |
| quercetin-3-O-galactoside (F2) | 52.94 | 174.17 | 0.47 ± 0.005 c | 0.24 ± 0.003 d | 0.52 ± 0.003 b | 0.57 ± 0.005 a | 0.20 ± 0.003 e | 0.02 ± 0.001 g | 0.01 ± 0.001 h | nd | nd | 0.05 ± 0.001 f |
| isoquercitrin (F3) | 58.42 | 192.52 | 0.22 ± 0.005 b | 0.32 ± 0.006 a | 0.18 ± 0.006 c | 0.21 ± 0.013 b | 0.08 ± 0.001 d | 0.01± 0.001 g | 0.05± 0.006 e | 0.02 ± 0.001 fg | 0.02 ± 0.003 fg | 0.03 ± 0.001 ef |
| kaempferol-3-O-glucuronide (F4) | 43.98 | 153.14 | 1.33 ± 0.001 b | 0.86 ± 0.001 d | 1.88 ± 0.001 a | 0.95 ± 0.001 c | 0.51 ± 0.001 g | 0.18 ± 0.001 i | 0.61 ± 0.001 f | 0.74 ± 0.001 e | 0.20 ± 0.001 h | 0.17 ± 0.001 i |
| kaempferol-3-O-glucoside (F5) glucoside (F5) | 53.85 | 187.37 | 0.18± 0.001 b | 0.20 ± 0.001 a | 0.12 ± 0.001 c | 0.08 ± 0.001 d | 0.05 ± 0.001 e | nd | 0.02 ± 0.002 f | nd | nd | nd |
| quercetin-3-O-rhamnoside (F6) rhamnosiderhamnoside (F6) | 62.48 | 199.32 | 0.23 ± 0.001 a | 0.11 ± 0.001 d | 0.20 ± 0.001 b | 0.19 ± 0.001 c | nd | 0.01 ± 0.001 g | 0.01 ± 0.001 f | 0.04 ± 0.001 e | nd | nd |
| kaempferol-3-O-rhamnoside (F7) | 64.13 | 211.81 | 2.30 ± 0.001 a | 1.39 ± 0.001 d | 1.78 ± 0.001 b | 1.50 ± 0.006 c | 0.68 ± 0.001 e | nd | 0.05 ± 0.001 g | 0.25 ± 0.001 f | nd | nd |
| arjunolic acid (T1) | 78.13 | 260.17 | 2.25 ± 0.001 b | 1.65 ± 0.0011 c | 2.70 ± 0.001 a | 1.60 ± 0.001 d | 1.47 ± 0.001 e | 0.02 ± 0.001 g | 0.07 ± 0.001 f | 0.07 ± 0.001 f | nd | 0.02 ± 0.001 g |
| cyclocaric acid B (T2) | 90.16 | 296.36 | 0.71 ± 0.001 c | 0.95 ± 0.001 b | 0.98 ± 0.001 a | 0.65 ± 0.001 d | 0.55 ± 0.001 e | 0.05± 0.004g h | 0.07 ± 0.001 f | 0.06 ± 0.001 g | 0.04 ± 0.002 h | 0.05 ± 0.001 gh |
| pterocaryoside B (T3) | 72.46 | 210.27 | 0.24 ± 0.001 d | 0.01 ± 0.001 e | 1.61 ± 0.008 b | 1.42 ± 0.002 c | 2.46 ± 0.002 a | nd | nd | nd | nd | nd |
| pterocaryoside A (T4) | 86.39 | 268.62 | 1.66 ± 0.007 d | 1.07 ± 0.004 e | 1.73 ± 0.003 c | 2.03 ± 0.002 b | 2.48 ± 0.006 a | nd | nd | nd | nd | nd |
| hederagenin (T5) | 72.82 | 232.30 | 0.56 ± 0.002 c | 0.45 ± 0.002 e | 0.81 ± 0.004 a | 0.70 ± 0.001 b | 0.52 ± 0.003 d | nd | nd | nd | nd | nd |
| oleanolic acid (T6) | 58.41 | 185.72 | 0.26 ± 0.002 c | 0.25 ± 0.001 c | 0.31 ± 0.001 a | 0.30 ± 0.002 b | 0.22 ± 0.002 d | 0.003 ± 0.0001 fg | 0.009 ± 0.0001 e | 0.007 ± 0.0002 ef | 0.001 ± 0.0001 g | 0.003 ± 0.0001 fg |
| total phenolic acids (TP) | - | - | 0.43 ± 0.014 e | 1.57 ± 0.154 a | 1.09 ± 0.023 b | 0.71 ± 0.034 d | 0.47 ± 0.007 e | 0.12 ± 0.003 f | 1.53 ± 0.026 a | 0.91 ± 0.003 c | 0.06 ± 0.003 f | 0.13 ± 0.009 f |
| total flavonoids (TF) | - | - | 6.88 ± 0.011 a | 4.70 ± 0.002 d | 6.80 ± 0.006 b | 5.90 ± 0.008 c | 2.30 ± 0.001 e | 0.55 ± 0.003 i | 1.99 ± 0.021 g | 2.03 ± 0.001 f | 0.52± 0.003 j | 0.60 ± 0.010 h |
| total triterpenoids (TT) | - | - | 5.69 ± 0.012 d | 4.39 ± 0.004 e | 8.15 ± 0.018 a | 6.70 ± 0.008 c | 7.70 ± 0.001 b | 0.08 ± 0.004 g | 0.15 ± 0.002 f | 0.13 ± 0.001 f | 0.05 ± 0.003 h | 0.07 ± 0.001 g |
| polysaccharides (Ps) | - | - | nd | nd | nd | nd | nd | 34.5 ± 2.02 c | 44.0 ± 5.54 b | 53.6 ± 4.05 a | 28.8 ± 4.91 cd | 28.2 ± 3.07 d |
| Treatment | Fasting blood glucose level (mmol /L) | ||||
|---|---|---|---|---|---|
| 0 day | 7 day | 14 day | 21 day | 28 day | |
| DC | 17.9 ± 0.54 a | 19.0 ± 0.46 a | 19.2 ± 0.75 a | 19.4 ± 0.82 a | 20.0 ± 0.73 a |
| NC | 6.6 ± 0.31 b | 6.2 ± 0.27 e | 6.3 ± 0.27 g | 6.1 ± 0.44 g | 6.1 ± 0.28 g |
| EL1 | 16.8 ± 0.43 a | 15.8 ± 0.69 cd | 14.9 ± 0.73 bcde | 12.0 ± 0.87 cde | 10.6 ± 0.48 de |
| EL2 | 16.8 ± 0.88 a | 15.5 ± 0.35 d | 14.2 ± 0.51 cdef | 14.3 ± 0.58 b | 12.8 ± 0.89 b |
| EL3 | 17.8 ± 0.40 a | 15.5 ± 0.42 d | 13.3 ± 0.95 ef | 10.7 ± 0.61 ef | 9.8 ± 0.45 ef |
| EL4 | 16.8 ± 1.10 a | 15.5 ± 0.82 d | 13.2 ± 0.93 ef | 10.9 ± 1.03 def | 10.4 ± 1.20 e |
| EL5 | 17.9 ± 1.02 a | 15.9 ± 0.71 cd | 14.1 ± 0.78 def | 13.2 ± 1.20 bc | 12.4 ± 1.06 b |
| AL1 | 17.4 ± 1.01 a | 16.6 ± 0.55b cd | 15.0 ± 1.10b cde | 13.7 ± 0.98 bc | 11.8 ± 1.10 bcd |
| AL2 | 17.5 ± 0.78 a | 16.3 ± 0.56b cd | 15.2 ± 0.87b cde | 12.7 ± 1.07 bcd | 11.8 ± 1.49 bcd |
| AL3 | 18.1 ± 0.60 a | 17.7 ± 1.63 ab | 15.6 ± 1.30 bcd | 13.5 ± 1.27 bc | 12.8 ± 1.35 b |
| AL4 | 17.4 ± 1.22 a | 18.4 ± 1.27 a | 16.5 ± 1.16 b | 12.4 ± 1.28 bcde | 10.8 ± 1.34 cde |
| AL5 | 17.1 ± 0.78 a | 17.3 ± 1.20 abc | 16.2 ± 0.70 bc | 13.1 ± 1.28 bc | 12.1 ± 1.42 bc |
| MHT | 17.4 ± 0.82 a | 15.0 ± 0.85 d | 12.6 ± 1.16 f | 9.2 ± 0.74 f | 8.5 ± 0.66 f |
| XKP | 17.8 ± 0.57 a | 15.7 ± 1.06 cd | 13.3 ± 1.74 ef | 10.8 ± 0.78 def | 9.5 ± 0.59 ef |
| Treatment | TC (mmol/L) | TG (mmol/L) | HDL-c (mmol/L) | LDL-c (mmol/L) |
|---|---|---|---|---|
| DC | 3.76 ± 0.19 a | 3.09 ± 0.49 a | 1.57 ± 0.25 c | 0.31 ± 0.05 a |
| NC | 2.64 ± 0.23 bcd | 1.48 ± 0.30 c | 1.87 ± 0.24 abc | 0.20 ± 0.04 cd |
| EL1 | 2.39 ± 0.25 cd | 1.21 ± 0.21 c | 1.95 ± 0.24 abc | 0.24 ± 0.04 abcd |
| EL2 | 2.08 ± 0.38 d | 1.19 ± 0.23 c | 2.11 ± 0.21 abc | 0.21 ± 0.03 bcd |
| EL3 | 2.08 ± 0.15 d | 1.15 ± 0.24 c | 2.34 ± 0.39 a | 0.18 ± 0.02 d |
| EL4 | 2.38 ± 0.21 cd | 1.02 ± 0.13 c | 2.23 ± 0.34 ab | 0.23 ± 0.03 abcd |
| EL5 | 2.57 ± 0.49 bcd | 1.21 ± 0.04 c | 2.05 ± 0.31 abc | 0.24 ± 0.04 abcd |
| AL1 | 2.66 ± 0.42 bcd | 1.45 ± 0.37 c | 1.66 ± 0.11 c | 0.28 ± 0.03 abc |
| AL2 | 2.97 ± 0.32 bc | 1.41 ± 0.29 c | 1.78 ± 0.18 bc | 0.28 ± 0.04 abc |
| AL3 | 3.03± 0.12 b | 1.56 ± 0.16 c | 1.74 ± 0.21 bc | 0.29 ± 0.05 ab |
| AL4 | 3.10 ± 0.42 b | 1.24 ± 0.12 c | 1.58 ± 0.16 c | 0.31 ± 0.04 a |
| AL5 | 2.95 ± 0.25 bc | 1.29 ± 0.28 c | 1.59 ± 0.08 c | 0.25 ± 0.04 abcd |
| MHT | 3.00 ± 0.19 b | 2.35± 0.29 b | 1.70 ± 0.24 bc | 0.23 ± 0.02 abcd |
| XKP | 3.12 ± 0.23 b | 2.36 ± 0.34 b | 1.93 ± 0.18 abc | 0.27 ± 0.03 abcd |
| Treatment | ALT (U/L) | AST (U/L) | TBIL (μmol/L) | CREA (μmol/L) | BUN (mmol/L) |
|---|---|---|---|---|---|
| DC | 52.8± 2.17 a | 145.6 ± 3.08 a | 7.50 ± 0.89 a | 25.8 ± 1.17 a | 17.3 ± 0.99 a |
| NC | 28.0 ± 1.84 e | 99.8 ± 6.87 fg | 1.00 ± 0.18 g | 14.7 ± 1.36 e | 9.80 ± 0.18 d |
| EL1 | 36.1 ± 2.63 cd | 113.3 ± 8.10 def | 1.20 ± 0.15 fg | 20.8 ± 0.75 bcd | 13.4 ± 0.86 b |
| EL2 | 35.9 ± 1.80 cd | 94.8 ± 8.59 g | 1.02 ± 0.02 g | 19.2 ± 1.94 cd | 12.6± 1.08 bc |
| EL3 | 35.1 ± 3.36 d | 94.5 ± 4.45 g | 1.19 ± 0.18 fg | 18.2 ± 2.13 d | 13.1 ± 1.26 bc |
| EL4 | 36.9 ± 3.35 bcd | 95.8 ± 9.05 g | 1.46 ± 0.16 fg | 21.0 ± 0.89 bcd | 11.0 ± 1.17 cd |
| EL5 | 36.2 ± 3.38 cd | 114.0 ± 7.66 de | 1.38 ± 0.18 fg | 21.0 ± 1.26 bcd | 12.7 ± 0.26 bc |
| AL1 | 40.3 ± 4.51 bcd | 130.2 ± 4.56 b | 1.47 ± 0.18 fg | 21.7 ± 1.86 bc | 14.7 ± 0.81 b |
| AL2 | 43.4 ± 6.36 b | 128.9 ± 5.30 bc | 2.03 ± 0.10 ef | 22.3 ± 1.21 b | 14.2 ± 0.69 b |
| AL3 | 41.6 ± 1.36 bcd | 123.6 ± 6.69 bcd | 2.57 ± 0.28 e | 22.8 ± 0.98 b | 14.3 ± 0.92 b |
| AL4 | 43.1± 5.16 b | 125.8 ± 4.16 bcd | 4.87 ± 0.17 bc | 21.8 ± 1.17 bc | 14.2 ± 1.17 b |
| AL5 | 40.0 ± 3.19 bcd | 115.5 ± 6.08 cde | 3.63 ± 0.21 d | 21.2 ± 0.98 bc | 14.2 ± 1.32 b |
| MHT | 42.4 ± 2.06 bc | 121.1± 6.06 bcde | 5.60 ± 0.55 b | 21.0 ± 1.67 bcd | 14.3 ± 0.76 b |
| XKP | 43.5 ± 2.39 b | 108.2 ± 8.57 efg | 4.41 ± 0.22 cd | 22.0 ± 1.79 bc | 13.9 ± 0.82 b |
| Sample code | Location | Latitude (N) | Longitude (E) | T (℃) | Altitude (m) | SH (h) | P (mm) | Soil index | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pH | C (%) | N (%) | P (%) | K (%) | Ca (%) | Mg (%) | ||||||||
| L1 | Jinzhongshan | 24°36′36″ | 104°57′00″ | 17.1 | 1798 | 1475 | 1200.0 | 3.97 | 5.15 | 0.44 | 0.35 | 0.55 | 0.06 | 0.33 |
| L2 | Muchuan | 28°58′00″ | 103°47′00″ | 12.9 | 1200 | 965.3 | 1533.2 | 3.81 | 3.58 | 0.27 | 0.08 | 0.98 | 0.19 | 0.22 |
| L3 | Wufeng | 30°17'00″ | 110°80'00″ | 16.7 | 688 | 1533 | 1893.9 | 4.38 | 4.18 | 0.27 | 0.12 | 1.44 | 0.39 | 0.41 |
| L4 | Meiwu | 27°46'00″ | 119°17'00″ | 16.5 | 678 | 1862 | 1600.0 | 4.48 | 6.46 | 0.45 | 0.22 | 2.03 | 0.18 | 0.22 |
| L5 | Suining | 26°22'24″ | 110°07'47″ | 16.7 | 862 | 1348 | 1320.0 | 4.61 | 3.17 | 0.38 | 0.13 | 1.28 | 0.22 | 0.22 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Cao, Y.; Fang, S.; Wang, T.; Yin, Z.; Shang, X.; Yang, W.; Fu, X. Antidiabetic Effect of Cyclocarya paliurus Leaves Depends on the Contents of Antihyperglycemic Flavonoids and Antihyperlipidemic Triterpenoids. Molecules 2018, 23, 1042. https://doi.org/10.3390/molecules23051042
Liu Y, Cao Y, Fang S, Wang T, Yin Z, Shang X, Yang W, Fu X. Antidiabetic Effect of Cyclocarya paliurus Leaves Depends on the Contents of Antihyperglycemic Flavonoids and Antihyperlipidemic Triterpenoids. Molecules. 2018; 23(5):1042. https://doi.org/10.3390/molecules23051042
Chicago/Turabian StyleLiu, Yang, Yanni Cao, Shengzuo Fang, Tongli Wang, Zhiqi Yin, Xulan Shang, Wanxia Yang, and Xiangxiang Fu. 2018. "Antidiabetic Effect of Cyclocarya paliurus Leaves Depends on the Contents of Antihyperglycemic Flavonoids and Antihyperlipidemic Triterpenoids" Molecules 23, no. 5: 1042. https://doi.org/10.3390/molecules23051042
APA StyleLiu, Y., Cao, Y., Fang, S., Wang, T., Yin, Z., Shang, X., Yang, W., & Fu, X. (2018). Antidiabetic Effect of Cyclocarya paliurus Leaves Depends on the Contents of Antihyperglycemic Flavonoids and Antihyperlipidemic Triterpenoids. Molecules, 23(5), 1042. https://doi.org/10.3390/molecules23051042