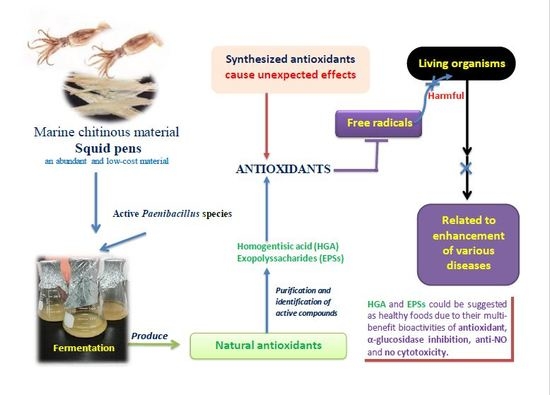
Production and Bioactivity-Guided Isolation of Antioxidants with α-Glucosidase Inhibitory and Anti-NO Properties from Marine Chitinous Materials
Abstract
:1. Introduction
2. Results and Discussion
2.1. Screening Suitable C/N Source for Antioxidant Production by Paenibacillus sp. TKU042
2.2. Antioxidant Production by Paenibacillus and Chitinolytic and/or Proteolytic Enzyme-Producing Strains
2.3. Optimization of Cultivation Conditions for Enhancement of Antioxidant Production
2.3.1. Optimization of Cultivation Time and Effect of Supplementary Air on Antioxidant Production
2.3.2. Optimization of Parameters to Achieve Greater Antioxidant Productivity
2.4. Isolation and Identification of Major Active Compounds from Fermented Product
2.5. Cytotoxicity, Anti-Imflammation and α-Glucosidase Inhibition of Crude Sample, Fraction, and Isolated Compounds
3. Materials and Methods
3.1. Materials
3.2. Bioactivity Assays
3.3. Optimization of Cultivation Conditions for Enhancement of Antioxidant Production
3.3.1. Optimization of Cultivation Time and Effect of Supplementary Air on Antioxidant Production
3.3.2. Optimization of Parameters to Achieve Greater Antioxidant Production
3.4. Enzymatic Assays, Total Sugars, and Reducing Sugars Detection
3.5. Experimental Procedures for Partial Purification and Identification of Major Active Components from Fermented Product
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mahapatra, S.; Banerjee, D. Evaluation of in vitro antioxidant potency of exopolysaccharide from endophytic Fusarium solani SD5. Int. J. Biol. Macromol. 2013, 53, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Luo, J.; Ye, H.; Sun, Y.; Lu, Z.; Zeng, X. Production, characterization and antioxidant activities in vitro of exopolysaccharides from endophytic bacterium Paenibacillus. polymyxa EJS-3. Carbohydr. Polym. 2009, 78, 275–281. [Google Scholar] [CrossRef]
- Liang, T.W.; Tseng, S.C.; Wang, S.L. Production and characterization of antioxidant properties of exopolysaccharides from Paenibacillus. mucilaginosus TKU032. Mar. Drugs 2016, 14, 40. [Google Scholar] [CrossRef] [PubMed]
- Al-Jaber, N.A.; Awaad, A.S.; Moses, J. Review on some antioxidant plants growing in Arab world. J. Saudi Chem. Soc. 2011, 15, 293–307. [Google Scholar] [CrossRef]
- Nguyen, Q.V.; Nguyen, N.H.; Wang, S.L.; Nguyen, V.B.; Nguyen, A.D. Free radical scavenging and antidiabetic activities of Euonymus laxiflorus Champ extract. Res. Chem. Intermed. 2017, 43, 5615–5624. [Google Scholar] [CrossRef]
- Nguyen, Q.V.; Nguyen, V.B.; Eun, J.B.; Wang, S.L.; Nguyen, D.H.; Tran, T.N.; Nguyen, A.D. Anti-oxidant and antidiabetic effect of some medicinal plants belong to Terminalia species collected in Dak Lak Province, Vietnam. Res. Chem. Intermed. 2016, 42, 5859–5871. [Google Scholar] [CrossRef]
- Sila, A.; Bougatef, A. Antioxidant peptides from marine by-products: Isolation, identification and application in food systems. A review. J. Funct. Foods 2016, 21, 10–26. [Google Scholar] [CrossRef]
- Chand, K.; Rajeshwari; Hiremathad, A.; Singh, M.; Santos, M A.; Keri, R.S. A review on antioxidant potential of bioactive heterocycle benzofuran: Natural and synthetic derivatives. Pharmacol. Rep. 2017, 69, 281–295. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.B.; Wang, S.L. New novel α-glucosdase inhibitors produced by microbial conversion. Process Biochem. 2018, 65, 228–232. [Google Scholar] [CrossRef]
- Dey, T.B.; Chakraborty, S.; Jain, K.K.; Sharma, A.; Kuhad, R.C. Antioxidant phenolics and their microbial production by submerged and solid state fermentation process: A review. Trends Food Sci. Technol. 2016, 53, 60–74. [Google Scholar]
- Wang, S.L.; Li, H.T.; Zhang, L.J.; Lin, Z.H.; Kuo, Y.H. Conversion of squid pen to homogentisic acid via Paenibacillus. sp. TKU036 and the antioxidant and anti-inflammatory activities of homogentisic acid. Mar. Drugs 2016, 14, 183. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Mao, W.; Tao, H.; Zhu, W.; Qi, X.; Chen, Y.; Li, H.; Zhao, C.; Yang, Y.; Hou, Y.; et al. Structural characterization and antioxidant properties of an exopolysaccharide produced by the mangrove endophytic fungus Aspergillus sp. Y16. Bioresour. Technol. 2011, 102, 8179–8184. [Google Scholar] [CrossRef] [PubMed]
- Confortin, F.G.; Marchetto, R.; Bettin, F.; Camassola, M.; Salvado, M.; Dillon, A.J. Production of Pleurotus. sajor-caju strain PS-2001 biomass in submerged culture. J. Ind. Microbiol. Biotechnol. 2008, 35, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Liu, H.; Wu, S.; Pang, L.; Jia, M.; Fan, K.; Jia, S.; Jia, L. Production and in vitro antioxidant activity of exopolysaccharide by a mutant, Cordyceps militaris SU5-08. Int. J. Biol. Macromol. 2012, 51, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Zhao, Z.; Chen, S.F.; Li, Y.Q. Optimization for the production of exopolysaccharide from Fomes. fomentarius in submerged culture and its antitumor effect in vitro. Bioresour. Technol. 2008, 99, 3187–3194. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.O.; Lim, J.M.; Joo, J.H.; Kim, S.W.; Hwang, H.J.; Choi, J.W.; Yun, J.W. Optimization of submerged culture condition for the production of mycelial biomass and exopolysaccharides by Agrocybe. cylindracea. Bioresour. Technol. 2005, 96, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Cho, E.J.; Oh, J.Y.; Chang, H.Y.; Yun, J.W. Production of exopolysaccharides by submerged mycelial culture of a mushroom Tremella fuciformis. J. Biotechnol. 2006, 127, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.M.; Kim, S.W.; Hwang, H.J.; Joo, J.H.; Kim, H.O.; Choi, J.W.; Yun, J.W. Optimization of medium by orthogonal matrix method for submerged mycelial culture and exopolysaccharide production in Collybia. maculata. Appl. Biochem. Biotechnol. 2004, 119, 159–170. [Google Scholar] [CrossRef]
- De Baets, S.; Du Laing, S.; Francois, C.; Vandamme, E.J. Optimization of exopolysaccharide production by Tremella mesenterica NRRL Y-6158 through implementation of fed-batch fermentation. J. Ind. Microbiol. Biotechnol. 2002, 29, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.H.; Chen, D.X.; Liu, J.W.; Liu, Z.L.; Wan, W.H.; Fang, N.; Xiao, Y.; Qi, Y.; Liang, Z.Q. Optimization of submerged culture requirements for the production of mycelial growth and exopolysaccharide by Cordyceps jiangxiensis JXPJ 0109. J. Appl. Microbiol. 2004, 96, 1105–1116. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.B.; Nguyen, A.D.; Kuo, Y.H.; Wang, S.L. Biosynthesis of α–glucosidase inhibitors by a newly isolated bacterium, Paenibacillus. sp. TKU042 and its effect on reducing plasma glucose in mouse model. Int. J. Mol. Sci. 2017, 18, 700. [Google Scholar]
- Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. Utilization of fishery processing by-product squid pens for α-glucosidase inhibitors production by Paenibacillus sp. Mar. Drugs 2017, 15, 274. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.D.; Huang, C.C.; Liang, T.W.; Nguyen, V.B.; Pan, P.S.; Wang, S.L. Production and purification of a fungal chitosanase and chitooligomers from Penicillium janthinellum D4 and discovery of the enzyme activators. Carbohydr. Polym. 2014, 108, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.W.; Chen, W.T.; Lin, Z.H.; Kuo, Y.H.; Nguyen, A.D.; Pan, P.S.; Wang, S.L. An amphiprotic novel chitosanase from Bacillus mycoides and its application in the production of chitooligomers with their antioxidant and anti-inflammatory evaluation. Int. J. Mol. Sci. 2016, 17, 1302. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.W.; Wang, S.L. Recent advances in exopolysaccharides from Paenibacillus spp.: Production, isolation, structure, and bioactivities. Mar. Drugs 2015, 13, 1847–1863. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.W.; Wu, C.C.; Cheng, W.T.; Chen, Y.C.; Wang, C.L.; Wang, I.L.; Wang, S.L. Exopolysaccharides and antimicrobial biosurfactants produced by Paenibacillus. macerans TKU029. Appl. Biochem. Biotechnol. 2014, 172, 933–950. [Google Scholar] [CrossRef] [PubMed]
- Liang, T.W.; Lo, B.C.; Wang, S.L. Chitinolytic bacteria-assisted conversion of squid pen and its effect on dyes and adsorption. Mar. Drugs 2015, 13, 4576–4593. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Chen, S.Y.; Yen, Y.H.; Liang, T.W. Utilization of chitinous materials in pigment adsorption. Food Chem. 2012, 135, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.F.; Hou, M.H.; Wang, T.S.; Chyau, C.C.; Chen, Y.Y. Enhanced antioxidant activity of Monascus pilosus fermented products by addition of ginger to the medium. Food Chem. 2009, 116, 915–922. [Google Scholar] [CrossRef]
- Miyake, Y.; Fukumoto, S.; Okada, M.; Sakaida, K.; Nakamura, Y.; Osawa, T. Antioxidative catechol lignans converted from sesamin and sesaminol triglucoside by culturing with Aspergillus. J. Agric. Food Chem. 2005, 53, 22–27. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.C.; Chang, C.T.; Chao, W.W.; Lin, C.F.; Chou, S.T. Antioxidative activity and safety of the 50% ethanolic extract from red bean fermented by Bacillus subtilis IMR-NK1. J. Agric. Food Chem. 2002, 50, 2454–2458. [Google Scholar] [CrossRef] [PubMed]
- Dyah, H.W.; Joe, A.V.; Severino, S.P. Mathematical modeling of the development of antioxidant activity in soybeans fermented with Aspergillus oryzae and Aspergillus awamori in the solid state. J. Agric. Food Chem. 2009, 57, 540–544. [Google Scholar]
- Wang, C.L.; Huang, T.H.; Liang, T.W.; Wang, S.L. Production and characterization of exopolysaccharides and antioxidant from Paenibacillus. sp. TKU023. New Biotechnol. 2011, 28, 559–565. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Luo, J.; Ye, H.; Sun, Y.; Lu, Z.; Zeng, X. In vitro and in vivo antioxidant activity of exopolysaccharides from endophytic bacterium Paenibacillus polymyxa EJS-3. Carbohydr. Polym. 2010, 82, 1278–1283. [Google Scholar] [CrossRef]
- Liu, J.; Luo, J.; Ye, H.; Zeng, X. Preparation, antioxidant and antitumor activities in vitro of different derivatives of levan from endophytic bacterium Paenibacillus. polymyxa EJS-3. Food Chem. Toxicol. 2012, 50, 767–772. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.E.; Kwon, S.Y.; Park, J.M. Biocontrol activity of Paenibacillus. polymyxa AC-1 against Pseudomonas syringae and its interaction with Arabidopsis thaliana. Microbiol. Res. 2016, 185, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Kluyver, A.J.; van Zijp, J.C.M. The production of homogentisic acid out of phenylacetic acid by Aspergillus niger. Antonie Leeuwenhoek 1951, 17, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Kotob, S.I.; Coon, S.L.; Quintero, E.J.; Weiner, R.M. Homogentisic acid is the primary precursor of melanin synthesis in Vibrio cholerae, a hyphomonas strain, and Shewanella. colwelliana. Appl. Environm. Microbiol. 1995, 61, 1620–1622. [Google Scholar]
- Carreira, A.; Ferreira, L.M.; Loureiro, V. Brown pigments produced by Yarrowia. lipolytica result from extracellular accumulation of homogentisic acid. Appl. Environm. Microbiol. 2001, 67, 3463–3468. [Google Scholar] [CrossRef] [PubMed]
- Chung, M.J.; Park, J.K.; Park, Y.I. Anti-inflammatory effects of low-molecular weight chitosan oligosaccharides in IgE-antigen complex-stimulated RBL-2H3 cells and asthma model mice. Int. Immunopharmcol. 2012, 12, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.L.; Yeh, P.Y. Production of a surfactant- and solvent- stable alkaliphilic protease by bioconversion of shrimp shell wastes fermented by Bacillus subtilis TKU007. Process Biochem. 2006, 41, 1545–1552. [Google Scholar] [CrossRef]
- Nguyen, V.B.; Nguyen, Q.V.; Nguyen, A.D.; Wang, S.L. Porcine pancreatic α-amylase inhibitors from Euonymus laxiflorus Champ. Res. Chem. Intermed. 2017, 43, 259–269. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds including HGA (purified FSPP-2.8.3) and EPS (FSPP-2.13) isolated from FSPP are available from the authors. |





| No. | Bacterial Strain | DPPH Radical Scavenging Activity | |
|---|---|---|---|
| (%) | IC50 (µg/mL) | ||
| 1 | Paenibacillus sp. TKU042 | 94 | 124 ± 13.9 C |
| 2 | Paenibacillus sp. TKU037 | 93 | 138 ± 16.2 C |
| 3 | Paenibacillus sp. TKU036 (positive strain) | 87 | 143 ± 10.3 C |
| 4 | Paenibacillus mucilaginosus TKU032 | 83 | 169 ± 14.2 C |
| 5 | Paenibacillus macerans TKU029 | 88 | 170 ± 20.3 C |
| 6 | Bacillus sp. TKU004 | 56 | 2600 ± 346 B |
| 7 | Bacillus cereus TKU028 | 45 | 3000 ± 288 AB |
| 8 | Bacillus mycoides TKU038 | 44 | 3000 ± 173 AB |
| 9 | Lactobacillus paracasei subsp paracasei TKU010 | 57 | 2700 ± 115 B |
| Control (medium only) | 40 | 3500 ± 289 A | |
| α-Tocopherol (commercial antioxidant) | 95 | 30 ± 5.8 C | |
| Purification Steps | Components | DPPH Radical Scavenging Activity | ||
|---|---|---|---|---|
| IC50 (µg/mL) | Specific Activity (U/mg) | Activity Folds Enhancement | ||
| Crude sample | FSPP | 172 ± 15.2 | 5.8 | 1 |
| Diaion opened column | FSPP-2 | 80 ± 7.9 | 12.5 | 2.2 |
| Silica opened column | FSPP-2.8 | 19 ± 2.1 | 52.6 | 9.1 |
| FSPP-2.13 | 30 ± 1.9 | 33.3 | 5.7 | |
| preparative HPLC | FSPP-2.8.3 | 8.1 ± 0.71 | 123.5 | 21.3 |
| Recycling | FSPP-2.8.3 (HGA) | 5.4 ± 0.62 | 185.2 | 31.9 |
| Positive control | α-tocopherol | 24 ± 1.12 | ||
| Components | Cell Viability (%) | α-Glucosidase Inhibition IC50 (µg/mL) |
|---|---|---|
| FSPP | 91.42 ± 3.87 B | 275 ± 11.2 b |
| FSPP-2 | 103.05 ± 1.97 A | 457 ± 27.3 b |
| FSPP-2.8.3 (HGA) | 91.07 ± 2.37 B | 215 ± 15.3 b |
| FSPP-2.13 (EPS) | 95.80 ± 2.61 B | — |
| Acarbose (positive aGI) | 1324 ± 96.3 a | |
| Least Significant Difference (α = 0.1) | 5.5628 | 269.31 |
| Coefficient of variation | 1.927574 | 15.66989 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, V.B.; Nguyen, T.H.; Doan, C.T.; Tran, T.N.; Nguyen, A.D.; Kuo, Y.-H.; Wang, S.-L. Production and Bioactivity-Guided Isolation of Antioxidants with α-Glucosidase Inhibitory and Anti-NO Properties from Marine Chitinous Materials. Molecules 2018, 23, 1124. https://doi.org/10.3390/molecules23051124
Nguyen VB, Nguyen TH, Doan CT, Tran TN, Nguyen AD, Kuo Y-H, Wang S-L. Production and Bioactivity-Guided Isolation of Antioxidants with α-Glucosidase Inhibitory and Anti-NO Properties from Marine Chitinous Materials. Molecules. 2018; 23(5):1124. https://doi.org/10.3390/molecules23051124
Chicago/Turabian StyleNguyen, Van Bon, Thi Hanh Nguyen, Chien Thang Doan, Thi Ngoc Tran, Anh Dzung Nguyen, Yao-Haur Kuo, and San-Lang Wang. 2018. "Production and Bioactivity-Guided Isolation of Antioxidants with α-Glucosidase Inhibitory and Anti-NO Properties from Marine Chitinous Materials" Molecules 23, no. 5: 1124. https://doi.org/10.3390/molecules23051124
APA StyleNguyen, V. B., Nguyen, T. H., Doan, C. T., Tran, T. N., Nguyen, A. D., Kuo, Y. -H., & Wang, S. -L. (2018). Production and Bioactivity-Guided Isolation of Antioxidants with α-Glucosidase Inhibitory and Anti-NO Properties from Marine Chitinous Materials. Molecules, 23(5), 1124. https://doi.org/10.3390/molecules23051124