Effect of Microwave Vacuum Drying on the Drying Characteristics, Color, Microstructure, and Antioxidant Activity of Green Coffee Beans
Abstract
:1. Introduction
2. Results and Discussion
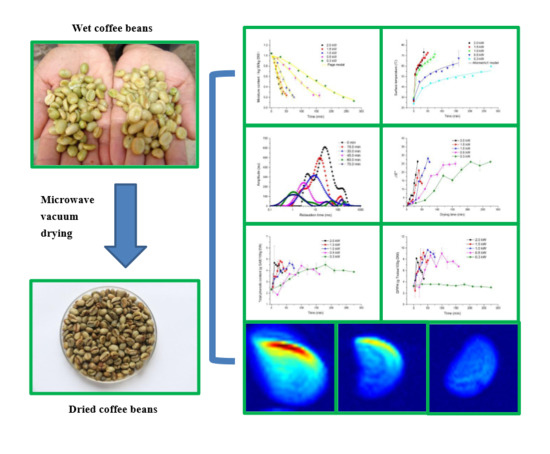
2.1. Drying Characteristics and Deff
2.2. Surface Temperature Rise Characteristics
2.3. Drying Kinetic and Surface Temperature Rise Kinetics
2.4. Differential Scanning Calorimetry Analysis
2.5. Water State Determination by Time-Domain Nuclear Magnetic Resonance
2.6. NMR Imaging
2.7. Color Analysis
2.8. Infrared Thermal Imaging
2.9. Scanning Electron Microscope Analysis
2.10. Total Phenolic Content Analysis
2.11. Antioxidant Activity Determinations
3. Materials and Methods
3.1. Materials
3.2. MVD
3.3. Mathematical Modeling
3.4. The Effective Moisture Diffusion Coefficient Deff
3.5. Surface Temperature
3.6. Color
3.7. Infrared Thermal Imaging
3.8. Differential Scanning Calorimetry
3.9. NMR
3.10. Scanning Electron Microscopy
3.11. Analysis of the TPC and Antioxidant Activity
3.11.1. Extraction of Phenolic Compounds
3.11.2. TPC Determination
3.11.3. Determination of DPPH Radical-Scavenging Activity
3.11.4. FRAP Determination
3.11.5. Determination of the ABTS Antioxidant Activity
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Alves, L.C.; De Magahäes, D.M.; Labate, M.T.V.; Gonzalez, S.G.; Labate, C.A.; Domingues, D.S.; Sera, T.; Vieira, L.G.E.; Pereira, L.F.P. Differentially accumulated proteins in Coffea arabica seeds during perisperm tissue development and their relationship to coffee grain size. J. Agric. Food Chem. 2016, 64, 1635–1647. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.J.; Tan, L.H.; Zhao, J.P.; Hu, R.S.; Lu, M.Q. Characterization of fatty acid, amino acid and volatile compound compositions and bioactive components of seven coffee (Coffea robusta) cultivars grown in Hainan Province, China. Molecules 2015, 20, 16687–16708. [Google Scholar] [CrossRef] [PubMed]
- Knopp, S.; Bytof, G.; Selmar, D. Influence of processing on the content of sugars in green Arabica coffee beans. Eur. Food Res. Technol. 2006, 223, 195. [Google Scholar] [CrossRef]
- Joët, T.; Laffargue, A.; Descroix, F.; Doulbeau, S.; Bertrand, B.; de kochko, A.; Dussert, S. Influence of environmental factors, wet processing and their interactions on the biochemical composition of green Arabica coffee beans. Food Chem. 2010, 118, 693–701. [Google Scholar] [CrossRef]
- Zhao, Y.T.; Jiang, Y.J.; Zheng, B.D.; Zhuang, W.J.; Zheng, Y.F.; Tian, Y.T. Influence of microwave vacuum drying on glass transition temperature, gelatinization temperature, physical and chemical qualities of lotus seeds. Food Chem. 2017, 228, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Pei, F.; Shi, Y.; Mariga, A.M.; Yang, W.J.; Tang, X.Z.; Zhao, L.Y.; An, X.X.; Hu, Q.H. Comparison of freeze-drying and freeze-drying combined with microwave vacuum drying methods on drying kinetics and rehydration characteristics of button mushroom (Agaricus bisporus) slices. Food Bioprocess Technol. 2014, 7, 1629–1639. [Google Scholar] [CrossRef]
- Mousa, N.; Farid, M. Microwave vacuum drying of banana slices. Dry. Technol. 2002, 20, 2055–2066. [Google Scholar] [CrossRef]
- Darvishi, H.; Asl, A.R.; Asghari, A.; Azadbakht, M.; Najafi, G.; Khodaei, J. Study of the drying kinetics of pepper. J. Saudi Soc. Agric. Sci. 2014, 13, 130–138. [Google Scholar] [CrossRef]
- Suriani, M.J.; Ali, A.; Khalina, A.; Sapuan, S.M.; Abdullah, S. Detection of defects in kenaf/epoxy using infrared thermal imaging technique. Procedia Chem. 2012, 4, 172–178. [Google Scholar] [CrossRef]
- Moss, J.R.; Otten, L. A relationship between colour development and moisture content during roasting of peanuts. Can. Inst. Food Sci. Technol. J. 1989, 22, 34–39. [Google Scholar] [CrossRef]
- Tian, Y.T.; Zhao, Y.T.; Huang, J.J.; Zeng, H.L.; Zheng, B.D. Effects of different drying methods on the product quality and volatile compounds of whole shiitake mushrooms. Food Chem. 2016, 197, 714–722. [Google Scholar] [CrossRef] [PubMed]
- Babova, O.; Occhipinti, A.; Maffei, M.E. Chemical partitioning and antioxidant capacity of green coffee (Coffea arabica and Coffea canephora) of different geographical origin. Phytochemistry 2016, 123, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Méndez-Lagunas, L.; Rodríguez-Ramírez, J.; Cruz-Gracida, M.; Sandoval-Torres, S.; Barriada-Bernal, G. Convective drying kinetics of strawberry (Fragaria ananassa): Effects on antioxidant activity, anthocyanins and total phenolic content. Food Chem. 2017, 230, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Naidu, M.M.; Sulochanamma, G.; Sampathu, S.R.; Srinivas, P. Studies on extraction and antioxidant potential of green coffee. Food Chem. 2008, 107, 377–384. [Google Scholar] [CrossRef]
- Patras, A.; Brunton, N.; Da Pieve, S.; Butler, F.; Downey, G. Effect of thermal and high pressure processing on antioxidant activity and instrumental colour of tomato and carrot purées. Innov. Food Sci. Emerg. Technol. 2009, 10, 16–22. [Google Scholar] [CrossRef]
- Patras, A.; Tiwari, B.K.; Brunton, N.P.; Butler, F. Modelling the effect of different sterilisation treatments on antioxidant activity and colour of carrot slices during storage. Food Chem. 2009, 114, 484–491. [Google Scholar] [CrossRef]
- Zielinska, M.; Michalska, A. Microwave-assisted drying of blueberry (Vaccinium corymbosum L.) fruits: Drying kinetics, polyphenols, anthocyanins, antioxidant capacity, colour and texture. Food Chem. 2016, 212, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhang, M.; Mujumdar, A.S.; Lim, R.X. Analysis of temperature distribution and SEM images of microwave freeze drying banana chips. Food Bioprocess Technol. 2013, 6, 1144–1152. [Google Scholar] [CrossRef]
- Chen, Q.Q.; Bi, J.F.; Wu, X.Y.; Yi, J.Y.; Zhou, L.Y.; Zhou, Y.H. Drying kinetics and quality attributes of jujube (Zizyphus jujuba Miller) slices dried by hot-air and short-and medium-wave infrared radiation. LWT Food Sci. Technol. 2015, 64, 759–766. [Google Scholar] [CrossRef]
- Rocculi, P.; Sacchetti, G.; Venturi, L.; Cremonini, M.; Rosa, M.D.; Pittia, P. Role of water state and mobility on the antiplasticization of green and roasted coffee beans. J. Agric. Food Chem. 2011, 59, 8265–8271. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros, L.F.; Teixeira, J.A.; Mussatto, S.I. Chemical, functional, and structural properties of spent coffee grounds and coffee silverskin. Food Bioprocess Technol. 2014, 7, 3493–3503. [Google Scholar] [CrossRef] [Green Version]
- Marcone, M.F.; Wang, S.N.; Albabish, W.; Nie, S.P.; Somnarain, D.; Hill, A. Diverse food-based applications of nuclear magnetic resonance (NMR) technology. Food Res. Int. 2013, 51, 729–747. [Google Scholar] [CrossRef]
- Xin, Y.; Zhang, M.; Adhikari, B. Effect of trehalose and ultrasound-assisted osmotic dehydration on the state of water and glass transition temperature of broccoli (Brassica oleracea L. var. botrytis L.). J. Food Eng. 2013, 119, 640–647. [Google Scholar] [CrossRef]
- Duce, S.L.; Hall, L.D. Visualization of the hydration of food by nuclear magnetic resonance imaging. J. Food Eng. 1995, 26, 251–257. [Google Scholar] [CrossRef]
- Horuz, E.; Bozkurt, H.; Karataş, H.; Maskan, M. Effects of hybrid (microwave-convectional) and convectional drying on drying kinetics, total phenolics, antioxidant capacity, vitamin C, color and rehydration capacity of sour cherries. Food Chem. 2017, 230, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Wojdyło, A.; Figiel, A.; Lech, K.; Nowicka, P.; Oszmiański, J. Effect of convective and vacuum–microwave drying on the bioactive compounds, color, and antioxidant capacity of sour cherries. Food Bioprocess Technol. 2014, 7, 829–841. [Google Scholar] [CrossRef]
- Zheng, M.Y.; Xia, Q.L.; Lu, S.M. Study on drying methods and their influences on effective components of loquat flower tea. LWT Food Sci. Technol. 2015, 63, 14–20. [Google Scholar] [CrossRef]
- Velioglu, Y.S.; Mazza, G.; Gao, L.; Oomah, B.D. Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J. Agric. Food Chem. 1998, 46, 4113–4117. [Google Scholar] [CrossRef]
- Rivera, W.; Velasco, X.; Gálvez, C.; Rincón, C.; Rosales, A.; Arango, P. Effect of the roasting process on glass transition and phase transition of Colombian Arabic coffee beans. Procedia Food Sci. 2011, 1, 385–390. [Google Scholar] [CrossRef]
- Soto, C.; Caballero, E.; Pérez, E.; Zúniga, M.E. Effect of extraction conditions on total phenolic content and antioxidant capacity of pretreated wild Peumus boldus leaves from Chile. Food Bioprod. Process. 2014, 92, 328–333. [Google Scholar] [CrossRef]
- Dong, W.J.; Ni, Y.N.; Kokot, S. A near-infrared reflectance spectroscopy method for direct analysis of several chemical components and properties of fruit, for example, Chinese hawthorn. J. Agric. Food Chem. 2013, 61, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Mahzir, K.A.M.; Gani, S.S.A.; Zaidan, U.H.; Halmi, M.I.E. Development of Phaleria macrocarpa (scheff.) Boerl fruits using response surface methodology focusd on phenolics, flavonoids and antioxidant properties. Molecules 2018, 23, 724. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, G.; Cleland, J. Modelling marital fertility by age and duration: An empirical appraisal of the Page model. Popul. Stud. 1988, 42, 241–257. [Google Scholar] [CrossRef]
- Midilli, A.; Kucuk, H.; Yapar, Z. A new model for single-layer drying. Dry. Technol. 2002, 20, 1503–1513. [Google Scholar] [CrossRef]
- Xanthopoulos, G.; Oikonomou, N.; Lambrinos, G. Applicability of a single-layer drying model to predict the drying rate of whole figs. J. Food Eng. 2007, 81, 553–559. [Google Scholar] [CrossRef]
- Contini, D.; Martelli, F.; Zaccanti, G. Photon migration through a turbid slab described by a model based on diffusion approximation. I. Theory. Appl. Opt. 1997, 36, 4587–4599. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.F.; Sun, J.H.; Liao, X.J.; Chen, F.; Zhao, G.H.; Wu, J.H.; Hu, X.S. Mathematical modeling on hot air drying of thin layer apple pomace. Food Res. Int. 2007, 40, 39–46. [Google Scholar] [CrossRef]
- Mohapatra, D.; Rao, P.S. A thin layer drying model of parboiled wheat. J. Food Eng. 2005, 66, 513–518. [Google Scholar] [CrossRef]
Sample Availability: Not available. |





| Microwave Power (kW) | a | b | R2 | RMSE | χ2 | Deff (cm2/min) |
|---|---|---|---|---|---|---|
| 0.3 | 21.41 | 0.17 | 0.88 | 31.12 | 4.45 | 1.13 × 10−3 |
| 0.5 | 25.22 | 0.18 | 0.88 | 35.63 | 5.94 | 1.70 × 10−3 |
| 1.0 | 35.78 | 0.15 | 0.97 | 7.73 | 1.29 | 3.78 × 10−3 |
| 1.5 | 40.57 | 0.14 | 0.98 | 6.39 | 0.80 | 5.57 × 10−3 |
| 2.0 | 39.51 | 0.15 | 0.97 | 51.45 | 10.29 | 6.62 × 10−3 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, W.; Cheng, K.; Hu, R.; Chu, Z.; Zhao, J.; Long, Y. Effect of Microwave Vacuum Drying on the Drying Characteristics, Color, Microstructure, and Antioxidant Activity of Green Coffee Beans. Molecules 2018, 23, 1146. https://doi.org/10.3390/molecules23051146
Dong W, Cheng K, Hu R, Chu Z, Zhao J, Long Y. Effect of Microwave Vacuum Drying on the Drying Characteristics, Color, Microstructure, and Antioxidant Activity of Green Coffee Beans. Molecules. 2018; 23(5):1146. https://doi.org/10.3390/molecules23051146
Chicago/Turabian StyleDong, Wenjiang, Ke Cheng, Rongsuo Hu, Zhong Chu, Jianping Zhao, and Yuzhou Long. 2018. "Effect of Microwave Vacuum Drying on the Drying Characteristics, Color, Microstructure, and Antioxidant Activity of Green Coffee Beans" Molecules 23, no. 5: 1146. https://doi.org/10.3390/molecules23051146
APA StyleDong, W., Cheng, K., Hu, R., Chu, Z., Zhao, J., & Long, Y. (2018). Effect of Microwave Vacuum Drying on the Drying Characteristics, Color, Microstructure, and Antioxidant Activity of Green Coffee Beans. Molecules, 23(5), 1146. https://doi.org/10.3390/molecules23051146