Detection and Identification of Estrogen Based on Surface-Enhanced Resonance Raman Scattering (SERRS)
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.1.1. Preparation of Silver Nanoparticles
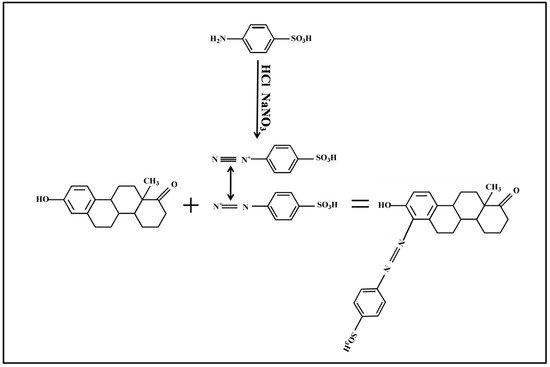
2.1.2. Pauly’s Reagent and Coupling Reaction
2.1.3. SERS Measurement
2.1.4. Characterization Methods
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kohei, T.; Rintaro, K.; Xiaohui, L.; Kaoru, U.; Tatsuya, K.; Kenji, W.; Tohru, O.; Hiroshi, N.; Yasuyuki, S. Design and Synthesis of Benzoacridines as Estrogenic and Anti-Estrogenic Agents. Bioorg. Med. Chem. 2017, 25, 5216–5237. [Google Scholar] [CrossRef]
- Engler-Chiurazzi, E.B.; Brown, C.M.; Povroznik, J.M.; Simpkins, J.W. Estrogens as neuroprotectants: Estrogenic actions in the context 3 of cognitive aging and brain injury. Prog. Neurobiol. 2017, 157, 188–211. [Google Scholar] [CrossRef] [PubMed]
- Ciubuc, J.D.; Bennet, K.E.; Qiu, C.; Alonzo, M.; Durrer, W.G.; Manciu, F.S. Raman Computational and Experimental Studies of Dopamine Detection. Biosensors 2017, 17, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Manciu, F.S.; Ciubuc, J.D.; Sundin, E.M.; Qiu, C.; Bennet, K.E. Analysis of Serotonin Molecules on Silver Nanocolloids—A Raman Computational and Experimental Study. Sensors 2017, 17, 1471–1482. [Google Scholar] [CrossRef] [PubMed]
- Engler-Chiurazzi, E.B.; Covey, D.F.; Simpkins, J.W. A novel mechanism of non-feminizing estrogens in neuroprotection. Exp. Gerontol. 2017, 94, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Thongon, N.; Boonmuen, N.; Suksen, K.; Wichit, P.; Chairoungdua, A.; Tuchinda, P.; Suksamrarn, A.; Winuthayanon, W.; Piyachaturawat, P. Selective Estrogen Receptor Modulator (SERM)-like activities of Diarylheptanoid, a Phytoestrogen from Curcuma comosa, in Breast Cancer Cells, Pre-Osteoblast Cells, and Rat Uterine Tissues. J. Agric. Food Chem. 2017, 65, 3490–3496. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wu, C.; Zhang, L.; Zhang, J. Ultraperformance Liquid Chromatography-Tandem Mass Spectrometry Method for Profiling Ketolic and Phenolic Sex Steroids Using an Automated Injection Program Combined with Diverter Valve Switch and Step Analysis. Anal. Chem. 2016, 88, 7878–7884. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Kuklenyik, Z.; Needham, L.L.; Calafat, A.M. Automated On-Line Column-Switching HPLC-MS/MS Method with Peak Focusing for the Determination of Nine Environmental Phenols in Urine. Anal. Chem. 2005, 77, 5407–5413. [Google Scholar] [CrossRef] [PubMed]
- Jaque, J.; Macdonald, H.; Brueggmann, D.; Patel, S.K.; Azen, C.; Clarke, N.; Stanczyk, F.Z. Deficiencies in immunoassay methods used to monitor serum Estradiol levels during aromatase inhibitor treatment in postmenopausal breast cancer patients. SpringerPlus 2013, 2, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Önder, F.C.; Mehmet, A.Y.; Sarker, S.D. Comparative Study of Antioxidant Properties and Total Phenolic Content of the Extracts of Humulus lupulus L. and Quantification of Bioactive Components by LC-MS/MS and GC-MS. J. Agric. Food Chem. 2013, 61, 10498–10506. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Diaz, I.; Zafra-Gomez, A.; Ballesteros, O.; Navea, N.; Navalon, A.; Fernandez, M.F.; Olea, N.; Vilchez, J.L. Determination of Bisphenol A and its chlorinated derivatives in placental tissue samples by liquid chromatography-tandem mass spectrometry. J. Chromatogr. B 2010, 878, 3363–3369. [Google Scholar] [CrossRef] [PubMed]
- Boggs, A.S.; Bowden, J.A.; Galligan, T.M.; Guillette, L.J.; Kucklick, J.R. Development of a multi-class steroid hormone screening method using Liquid Chromatography/Tandem Mass Spectrometry (LC-MS/MS). Anal. Bioanal. Chem. 2016, 408, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Čelić, M.; Insa, S.; Škrbić, B.; Petrović, M. Development of a sensitive and robust online dual column liquid chromatography-tandem mass spectrometry method for the analysis of natural and synthetic estrogens and their conjugates in river water and wastewater. Anal. Bioanal. Chem. 2017, 409, 5427–5440. [Google Scholar] [CrossRef] [PubMed]
- Geisler, J.; Berntsen, H.; Lonning, P.E. A novel HPLC-RIA method for the simultaneous detection of estrone, estradiol and Estrone sulphate levels in breast cancer tissue. J. Steroid Biochem. Mol. Biol. 2000, 72, 259–264. [Google Scholar] [CrossRef]
- Meulenaer, B.D.; Baert, K.; Lanckriet, H.; Hoed, V.V.; Huyghebaert, A. Development of an Enzyme-Linked Immunosorbent Assay for Bisphenol A Using Chicken Immunoglobulins. J. Agric. Food Chem. 2002, 50, 5273–5282. [Google Scholar] [CrossRef] [PubMed]
- Hegnerova, K.; Piliarik, M.; Steinbachova, M.; Flegelova, Z.; Cernohorska, H.; Homola, J. Detection of bisphenol A using a novel surface plasmon resonance biosensor. J. Anal. Bioanal. Chem. 2010, 398, 1963–1966. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liang, O.; Zhang, W.; Schroeder, T.; Xie, Y.H. Ultra-Sensitive Graphene-Plasmonic Hybrid Platform for Label-Free Detection. Adv. Mater. 2013, 25, 4918–4924. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, T.; Yoo, S.M.; Yoon, I.; Lee, S.Y.; Kim, B. Patterned multiplex pathogen DNA detection by Au particle-on-wire SERS sensor. Nano Lett. 2010, 10, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Emory, S.R. Probing Single Molecules and Single Nanoparticles by Surface-Enhanced Raman Scattering. Science 1997, 275, 1102–1106. [Google Scholar] [CrossRef] [PubMed]
- Ross, M.B.; Ashley, M.J.; Schmucker, A.L.; Singamaneni, S.; Naik, R.R.; Schatz, G.C.; Mirkin, C.A. Structure-Function Relationships for Surface-Enhanced Raman Spectroscopy Active Plasmonic Paper. J. Phys. Chem. C 2016, 120, 20789–20797. [Google Scholar] [CrossRef]
- Lee, S.; Chon, H.; Lee, J.; Ko, J.; Chung, B.H.; Lim, D.W.; Choo, J. Rapid and sensitive phenotypic marker detection on breast cancer cells using surface-enhanced Raman scattering (SERS) imaging. Biosens. Bioelectron. 2014, 51, 238–243. [Google Scholar] [CrossRef] [PubMed]
- Laing, S.; Gracie, K.; Faulds, K. Multiplex in vitro detection using SERS. Chem. Soc. Rev. 2016, 45, 1901–1918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngo, H.T.; Wang, H.N.; Fales, A.M.; Vodinh, T. Plasmonic SERS biosensingnano chips for DNA detection. Anal. Bioanal. Chem. 2016, 408, 1773–1781. [Google Scholar] [CrossRef] [PubMed]
- Chao, J.; Cao, W.F.; Su, S.; Weng, L.X.; Song, S.P.; Fan, C.H.; Wang, L.H. Nanostructure-based surface-enhanced Raman scattering biosensors for nucleic acids and proteins. J. Mater. Chem. B 2016, 4, 1757–1769. [Google Scholar] [CrossRef]
- Rycenga, M.; Langille, M.R.; Personick, M.L.; Ozel, T.; Mirkin, C.A. Chemically isolating hot spots on concave nanocubes. Nano Lett. 2012, 12, 6218. [Google Scholar] [CrossRef] [PubMed]
- Han, X.X.; Zeng, J.; Dearmon, B.M.; Zheng, Y.Q.; Li, Q.G.; Xia, Y.N. Silver nanocrystals with concave surfaces and their optical and surface-enhanced Raman scattering properties. Angew. Chem. 2011, 50, 12542–12546. [Google Scholar] [CrossRef]
- Siiman, O.; Lepp, A.; Kerker, M. Combined surface-enhanced and resonance-Raman scattering from the aspartic acid derivative of methyl orange on colloidal silver. J. Phys. Chem. 1983, 87, 5319–5325. [Google Scholar] [CrossRef]
- Graeme, M.E.D.; Smith, W.E; Faulds, K.; Graham, D. Surface-enhanced Raman scattering (SERS) and surface-enhanced resonance Raman scattering (SERRS): A review of applications. Appl. Spectrosc. 2011, 65, 825–837. [Google Scholar] [CrossRef]
- Moore, B.D.; Stevenson, L.; Watt, A.; Flitsch, S.; Turner, N.J.; Cassidy, C.; Graham, D. Rapid and ultra-sensitive determination of enzyme activities using surface-enhanced resonance Raman scattering. Nat. Biotechnol. 2004, 22, 1133–1138. [Google Scholar] [CrossRef] [PubMed]
- Han, X.X.; Pienpinijtham, P.; Zhao, B.; Ozaki, Y. Coupling Reaction-Based Ultrasensitive Detection of Phenolic Estrogens Using Surface-Enhanced Resonance Raman Scattering. Anal. Chem. 2011, 83, 8582–8588. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.H.; Liu, R.X.; Deng, Z.Y.; Ge, G.L; Liu, Y.; Xie, L.M. Quantitative study of protein coronas on gold nanoparticles with different surface modifications. Nano Res. 2014, 7, 345–352. [Google Scholar] [CrossRef]
- Wu, Y.Q.; Zhao, B.; Xu, WQ.; Li, B.F. Near-Infrared Surface-Enhanced Raman Scattering Study of Ultrathin Films of Azobenzene-Containing Long-Chain Fatty Acids on a Silver Surface Prepared by Silver Mirror and Nitric Acid Etched Silver Foil Methods. Langmuir 1999, 15, 4625–4629. [Google Scholar] [CrossRef]
- Biswas, N.; Umapathy, S. Density Functional Calculations of Structures, Vibrational Frequencies, and Normal Modes of trans- and cis-Azobenzene. J. Phys. Chem. A 1997, 101, 5555–5566. [Google Scholar] [CrossRef]
- Stuart, C.M.; Frontiera, R.R.; Mathies, R.A. Excited-state structure and dynamics of cis- and trans-Azobenzene from resonance Raman intensity analysis. J. Phys. Chem. A 2007, 111, 12072–12080. [Google Scholar] [CrossRef] [PubMed]
- Wilson, H.W.; Macnamee, R.W.; Durig, J.R. Raman spectra of gases: 24-Phenol. J. Raman Spectrosc. 1981, 11, 252–255. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are not available from the authors. |







© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Chen, Y.; Zhang, Y.; Kou, Q.; Zhang, Y.; Wang, Y.; Chen, L.; Sun, Y.; Zhang, H.; Jung, Y.M. Detection and Identification of Estrogen Based on Surface-Enhanced Resonance Raman Scattering (SERRS). Molecules 2018, 23, 1330. https://doi.org/10.3390/molecules23061330
Liu Y, Chen Y, Zhang Y, Kou Q, Zhang Y, Wang Y, Chen L, Sun Y, Zhang H, Jung YM. Detection and Identification of Estrogen Based on Surface-Enhanced Resonance Raman Scattering (SERRS). Molecules. 2018; 23(6):1330. https://doi.org/10.3390/molecules23061330
Chicago/Turabian StyleLiu, Yang, Yue Chen, Yuanyuan Zhang, Qiangwei Kou, Yongjun Zhang, Yaxin Wang, Lei Chen, Yantao Sun, Honglin Zhang, and Young Mee Jung. 2018. "Detection and Identification of Estrogen Based on Surface-Enhanced Resonance Raman Scattering (SERRS)" Molecules 23, no. 6: 1330. https://doi.org/10.3390/molecules23061330
APA StyleLiu, Y., Chen, Y., Zhang, Y., Kou, Q., Zhang, Y., Wang, Y., Chen, L., Sun, Y., Zhang, H., & Jung, Y. M. (2018). Detection and Identification of Estrogen Based on Surface-Enhanced Resonance Raman Scattering (SERRS). Molecules, 23(6), 1330. https://doi.org/10.3390/molecules23061330