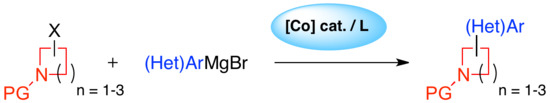
Cobalt-Catalyzed (Hetero)arylation of Saturated Cyclic Amines with Grignard Reagents
Abstract
:1. Introduction
2. Arylation of 4-Halopiperidines
3. Arylation of 3-Halopiperidines
4. Arylation of 3-Iodopyrrolidines
5. Arylation of 3-Iodoazetidines
6. Mechanistic Hypothesis
7. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Taylor, R.D.; MacCoss, M.; Lawson, A.D.G. Rings in Drugs. J. Med. Chem. 2014, 57, 5845. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.F.; Wink, M. Alkaloids: Biochemistry, Ecology and Medicinal Applications; Plenum: New York, NY, USA, 1998. [Google Scholar]
- Buckingham, J.; Baggaley, K.H.; Roberts, A.D.; Szabó, L.F. Dictionary of Alkaloids, 2nd ed.; CRC press: Boca Raton, FL, USA, 2009. [Google Scholar]
- De Risi, C.; Fanton, G.; Pollini, G.P.; Trapella, C.; Valente, F.; Zanirato, V. Recent advances in the stereoselective synthesis of trans 3,4-disubstituted piperidines: Applications to (-)-paroxetine. Tetrahedron Asymmetry 2008, 19, 131. [Google Scholar] [CrossRef]
- Wallace, D.J.; Baxter, C.A.; Brands, K.J.M.; Bremeyer, N.; Brewer, S.E.; Desmond, R.; Emerson, K.M.; Foley, J.; Fernandez, P.; Hu, W.; et al. Development of a fit-for-purpose large-scale synthesis of an oral PARP inhibitor. Org. Process. Res. Dev. 2011, 15, 831. [Google Scholar] [CrossRef]
- Brandi, A.; Cicchi, S.; Cordero, F.M. Novel syntheses of azetidines and azetidinones. Chem. Rev. 2008, 108, 3988. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.S.; D’hooghe, M.; De Kimpe, N. Azetidines, Azetines and Azetes: Monocyclic. In Comprehensive Heterocyclic Chemistry III; Stevens, C.V., Ed.; Elsevier: Oxford, UK, 2008; Volume 2, Chapter 2.01; p. 1. [Google Scholar]
- Couty, F.; Evano, G. Azetidines: New tools for the synthesis of nitrogen heterocycles. Synlett 2009, 19, 3053. [Google Scholar] [CrossRef]
- Buffat, M.G.P. Synthesis of piperidines. Tetrahedron 2004, 60, 1701. [Google Scholar] [CrossRef]
- Laschat, S.; Dickner, T. Stereoselective synthesis of piperidines. Synthesis 2000, 2000, 1781–1813. [Google Scholar] [CrossRef]
- Bott, T.M.; West, F.G. Preparation and synthetic applications of azetidines. Heterocycles 2012, 84, 223. [Google Scholar]
- Couty, F.; Drouillat, B.; Evano, G.; David, O. 2-cyanoazetidines and azetidinium ions: Scaffolds for molecular diversity. Eur. J. Org. Chem. 2013, 2013, 2045–2056. [Google Scholar] [CrossRef]
- Couty, F. Synthesis of azetidines. In Science of Synthesis: Houben-Weyl Methods of Molecular Transformations; Enders, D., Ed.; Georg Thieme Verlag: New York, NY, USA, 2009; Volume 40a, pp. 775–817. [Google Scholar]
- Stroman, N.A.; Sommer, S.; Fu, G.C. Hiyama reactions of activated and unactivated secondary alkyl halides catalysed by a nickel/norephedrine complex. Angew. Chem. Int. Ed. 2007, 46, 3556. [Google Scholar] [CrossRef] [PubMed]
- Powell, D.A.; Fu, G.C. Nickel-catalyzed cross-couplings of organosilicon reagents with unactivated secondary alkyl bromides. J. Am. Chem. Soc. 2004, 126, 7788. [Google Scholar] [CrossRef] [PubMed]
- González-Bobes, F.; Fu, G.C. Amino alcohols as ligands for nickel-catalyzed Suzuki reactions of unactivated alkyl halides, including secondary alkyl chlorides, with arylboronic acids. J. Am. Chem. Soc. 2006, 128, 5360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, C. Alkylations of arylboronic acids including difluoroethylation/trifluoroethylation via nickel-catalyzed Suzuki cross-coupling reaction. Adv. Synth. Catal. 2015, 357, 2721. [Google Scholar] [CrossRef]
- Magano, J.; Monfette, S. Development of an air-stable, broadly applicable nickel source for nickel-catalyzed cross-coupling. ACS Catal. 2015, 5, 3120. [Google Scholar] [CrossRef]
- Dander, J.E.; Weires, N.A.; Garg, N.K. Benchtop delivery of Ni(cod)2 using paraffin capsules. Org. Lett. 2016, 18, 3934. [Google Scholar] [CrossRef] [PubMed]
- Robbins, D.W.; Hartwig, J.F. Sterically controlled alkylation of arenes through iridium-catalyzed C-H borylation Angew. Chem. Int. Ed. 2013, 52, 933. [Google Scholar] [CrossRef] [PubMed]
- Vechorkin, O.; Proust, V.; Hu, X. Functional group tolerant Kumada-Corriu-Tamao coupling of nonactivated alkyl halides with aryl and heteroaryl nucleophiles: Catalysis by a nickel pincer complex permits the coupling of functionalized Grignard reagents. J. Am. Chem. Soc. 2009, 131, 9756. [Google Scholar] [CrossRef] [PubMed]
- Bica, K.; Gaertner, P. An iron-containing ionic liquid as recyclable catalyst for aryl Grignard cross-coupling of alkyl halides. Org. Lett. 2006, 8, 733. [Google Scholar] [CrossRef] [PubMed]
- Bauer, G.; Cheung, C.W.; Hu, X. Cross-coupling of nonactivated primary and secondary alkyl halides with aryl Grignard reagents catalysed by chiral iron pincer complexes. Synthesis 2015, 47, 1726. [Google Scholar]
- Despiau, C.F.; Dominey, A.P.; Harrowven, D.C.; Linclau, B. Total synthesis of (±)-Paroxetine by diastereoconvergent cobalt-catalyzed arylation. Eur. J. Org. Chem. 2014, 2014, 4335–4341. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Ito, S.; Matsuo, K.; Nakamura, E. Iron-catalyzed chemoselective cross-coupling of primary and secondary alkyl halides with arylzinc reagents. Synlett 2005, 11, 1794. [Google Scholar] [CrossRef]
- Hammann, J.M.; Haas, D.; Knochel, P. Cobalt-catalyzed Negishi cross-coupling reactions of (hetero)arylzinc reagents with primary and secondary alkyl bromides and iodides. Angew. Chem. Int. Ed. 2015, 54, 4478. [Google Scholar] [CrossRef] [PubMed]
- Pompeo, M.; Froese, R.D.J.; Hadei, N.; Organ, M.G. Pd-PEPPSI-IPentCl: A highly effective catalyst for the selective cross-coupling of secondary organozinc reagents. Angew. Chem. Int. Ed. 2012, 51, 11354. [Google Scholar] [CrossRef] [PubMed]
- Çalimsiz, S.; Organ, M.G. Negishi cross-coupling of secondary alkylzinc halides with aryl/heteroaryl halides using Pd-PEPPSI-IPent. Chem. Commun. 2011, 47, 5181. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Buchwald, S.L. Negishi coupling of secondary alkylzinc halides with aryl bromides and chlorides. J. Am. Chem. Soc. 2009, 131, 7532. [Google Scholar] [CrossRef] [PubMed]
- Seel, S.; Thaler, T.; Takatsu, K.; Zhang, C.; Zipse, H.; Straub, B.F.; Mayer, P.; Knochel, P. Highly diastereoselective arylations of substituted piperidines. J. Am. Chem. Soc. 2011, 133, 4774. [Google Scholar] [CrossRef] [PubMed]
- Melzig, L.; Gavryushin, A.; Knochel, P. Direct aminoalkylation of arenes and hetarenes via Ni-catalyzed Negishi cross-coupling reactions. Org. Lett. 2007, 9, 5529. [Google Scholar] [CrossRef] [PubMed]
- Corley, E.G.; Conrad, K.; Murry, J.A.; Savarin, C.; Holko, J.; Boice, G. Direct synthesis of 4-arylpiperidines via palladium/copper(I)-cocatalyzed Negishi coupling of a 4-piperidylzinc iodide with aromatic halides and triflates. J. Org. Chem. 2004, 69, 5120. [Google Scholar] [CrossRef] [PubMed]
- Billotte, S. Synthesis of C-substituted cyclic amines using azacycloalkyl organozinc reagents. Synlett 1998, 1998, 379–380. [Google Scholar] [CrossRef]
- Hofmayer, M.S.; Hammann, J.M.; Haas, D.; Knochel, P. Cobalt-catalyzed C(sp2)-C(sp3) cross-coupling reactions of diarylmanganese reagents with secondary alkyliodides. Org. Lett. 2016, 18, 6456. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Qian, Q.; Gong, H. Nickel-catalyzed reductive coupling of aryl halides with secondary alkyl bromides and allylic acetates. Org. Lett. 2012, 14, 3352. [Google Scholar] [CrossRef] [PubMed]
- Anka-Lufford, L.L.; Huihui, K.M.M.; Gower, N.J.; Ackerman, L.K.G.; Weix, D.J. Nickel-catalyzed cross-electrophile coupling with organic reductants in non-amide solvents. Chem. Eur. J. 2016, 22, 11564. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Liang, Z.; Qian, Q.; Lin, K. Nickel-catalyzed reductive alkylation of halogenated pyridines with secondary alkyl bromides. Synth. Commun. 2014, 44, 2999. [Google Scholar] [CrossRef]
- Molander, G.A.; Traister, K.M.; O’Neill, B.T. Reductive cross-coupling of nonaromatic, heterocyclic bromides with aryl and heteroaryl bromides. J. Org. Chem. 2014, 79, 5771. [Google Scholar] [CrossRef] [PubMed]
- Molander, G.A.; Traister, K.M.; O’Neill, B.T. Engaging nonaromatic, heterocyclic tosylates in reductive cross-coupling with aryl and heteroaryl bromides. J. Org. Chem. 2015, 80, 2907. [Google Scholar] [CrossRef] [PubMed]
- Millet, A.; Larini, P.; Clot, E.; Baudoin, O. Ligand-controlled β-selective C(sp3)-H arylation of N-Boc-piperidines. Chem. Sci. 2013, 4, 2241. [Google Scholar] [CrossRef]
- Malpass, J.R.; Handa, S.; White, R. Approaches to syn-7-substituted 2-azanorbornanes as potential nicotinic agonists; Synthesis of syn- and anti-isoepibatidine. Org. Lett. 2005, 7, 2759. [Google Scholar] [CrossRef] [PubMed]
- Hammann, J.M.; Steib, A.K.; Knochel, P. Cobalt-mediated diastereoselective cross-coupling reactions between cyclic halohydrins and arylmagnesium reagents. Org. Lett. 2014, 16, 6500. [Google Scholar] [CrossRef] [PubMed]
- Hammann, J.M.; Haas, D.; Steib, A.K.; Knochel, P. Highly diastereselective cobalt-mediated C(sp3)-C(sp2) cross-coupling reactions of cyclic halohydrins with (hetero)aryl Grignard reagents. Synthesis 2015, 47, 1461. [Google Scholar]
- Hammann, J.M.; Haas, D.; Tüllmann, C.-P.; Karaghiosoff, K.; Knochel, P. Diastereoselective cobalt-mediated cross-couplings of cycloalkyl iodides with alkynyl or (hetero)aryl Grignard reagents. Org. Lett. 2016, 18, 4778. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Le, C.C.; MacMillan, D.W.C. Silyl radical activation of alkyl halides in metallaphotoredox catalysis: A unique pathway for cross-elctrophile coupling. J. Am. Chem. Soc. 2016, 138, 8084. [Google Scholar] [CrossRef] [PubMed]
- Duncton, M.J.A.; Estiarte, M.A.; Tan, D.; Kaub, C.; O’Mahony, D.J.R.; Johnson, R.J.; Cox, M.; Edwards, W.T.; Wan, M.; Kincaid, J.; et al. Preparation of aryloxetanes and arylazetidines by use of an alkyl-aryl Suzuki coupling. Org. Lett. 2008, 10, 3259. [Google Scholar] [CrossRef] [PubMed]
- Armar, D.; Henkel, L.; Dib, J.; Rueping, M. Iron catalyzed cross-couplings of azetidines - application to the formal synthesis of a pharmacologically active molecule. Chem. Commun. 2015, 51, 2111. [Google Scholar]
- Honde, V.R.; O’Neill, B.T.; Buchwald, S.L. An improved system for the aqueous Lipshutz-Negishi cross-coupling of alkyl halides with aryl electrophiles. Angew. Chem. Int. Ed. 2016, 55, 1849. [Google Scholar]
- Schwertz, G.; Witschel, M.C.; Rottmann, M.; Bonnert, R.; Leartsakulpanich, U.; Chitnumsub, P.; Jaruwat, A.; Ittarat, W.; Schäfer, A.; Aponte, R.A.; et al. Antimalarial inhibitors targeting serine hydroxymethyltransferase (SHMT) with in vivo efficacy and analysis of their binding mode based on X-ray cocrystal structures. J. Med. Chem. 2017, 60, 4840. [Google Scholar] [CrossRef] [PubMed]
- Tarr, J.C.; Wood, M.R.; Noetzel, M.J.; Melancon, B.J.; Lamsal, A.; Luscombe, V.B.; Rodriguez, A.L.; Byers, F.W.; Chang, S.; Cho, H.P.; et al. Challenges in the development of an M4 PAM preclinical candidate: The discovery, SAR, and biological characterization of a series of azetidine-derived tertiary amides. Bioorg. Med. Chem. Lett. 2017, 27, 5179. [Google Scholar] [CrossRef] [PubMed]
- Gosmini, C.; Bégouin, J.-M.; Moncomble, A. Cobalt-catalyzed cross-coupling reactions. Chem. Commun. 2008, 3221. [Google Scholar] [CrossRef] [PubMed]
- Cahiez, G.; Moyeux, A. Cobalt-catalyzed cross-coupling reactions. Chem. Rev. 2010, 110, 1435. [Google Scholar] [CrossRef] [PubMed]
- Gosmini, C.; Moncomble, A. Cobalt-catalyzed cross-coupling reactions of aryl halides. Isr. J. Chem. 2010, 50, 568. [Google Scholar] [CrossRef]
- Barré, B.; Gonnard, L.; Campagne, R.; Reymond, S.; Marin, J.; Ciapetti, P.; Brellier, M.; Guérinot, A.; Cossy, J. Iron- and cobalt-catalyzed arylation of azetidines, pyrrolidines, and piperidines with Grignard reagents. Org. Lett. 2014, 16, 6160. [Google Scholar] [CrossRef] [PubMed]
- Gonnard, L.; Guérinot, A.; Cossy, J. Cobalt-catalyzed cross-coupling of 3- and 4-iodopiperidines with Grignard reagents. Chem. Eur. J. 2015, 21, 12797. [Google Scholar] [CrossRef] [PubMed]
- Piller, F.M.; Appukkuttan, P.; Gavryushin, A.; Helm, M.; Knochel, P. Convenient preparation of polyfunctional aryl magnesium reagents by a direct magnesium insertion in the presence of LiCl. Angew. Chem. Int. Ed. 2008, 47, 6802. [Google Scholar] [CrossRef] [PubMed]
- Wikström, H.; Sanchez, D.; Lindberg, P.; Hacksell, U.; Arvidsson, L.-E.; Johansson, A.M.; Thorberg, S.-O.; Nilsson, J.L.G.; Svensson, K.; Hjorth, S.; et al. Resolved 3-(3-hydroxyphenyl)-N-n-propylpiperidine and its analogue: Central dopamine receptor activity. J. Med. Chem. 1984, 27, 1030. [Google Scholar] [CrossRef] [PubMed]
- Tamminga, C.A.; Cascella, N.G.; Lahti, R.A.; Lindberg, M.; Carlsson, A. Pharmacologic properties of (-)-3PPP (preclamol) in man. J. Neural Transm. 1992, 88, 165. [Google Scholar] [CrossRef]
- Pirtosek, Z.; Merello, M.; Carlsson, A.; Stern, G. Preclamol. A “designer drug” in the treatment of advanced Parkinson’s disease. Adv. Neurol. 1996, 69, 535. [Google Scholar] [PubMed]
- Wakabayashi, K.; Yorimitsu, H.; Oshima, K. Cobalt-catalyzed tandem radical cyclization and cross-coupling reaction: Its application to benzyl-substituted heterocycles. J. Am. Chem. Soc. 2001, 123, 5374. [Google Scholar] [CrossRef] [PubMed]
- Ohmiya, H.; Wakabayashi, K.; Yorimitsu, H.; Oshima, K. Cobalt-catalyzed cross-coupling reactions of alkyl halides with aryl Grignard reagents and their application to sequential radical cyclization/cross-coupling reactions. Tetrahedron 2006, 62, 2207. [Google Scholar] [CrossRef]
- Ohmiya, H.; Yorimitsu, H.; Oshima, K. Cobalt-mediated cross-coupling reactions of primary and secondary alkyl halides with 1-(trimethylsilyl)ethenyl- and 2-trimethylsilylethynylmagnesium reagents. Org. Lett. 2006, 8, 3093. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, L.; Angibaud, P.; Stansfield, I.; Bonnet, P.; Meerpoel, L.; Reymond, S.; Cossy, J. Diastereoselective metal-catalyzed synthesis of C-aryl and C-vinyl glycosides. Angew. Chem. Int. Ed. 2012, 51, 11101. [Google Scholar] [CrossRef] [PubMed]













| Entry | 1 (X, PG) | [Co] | L [a] | 1/2 [b] | 2 (yield) [c] |
|---|---|---|---|---|---|
| 1 | 1a (I, Boc) | - | - | 1a/2a = 100:0 | - |
| 2 | 1a (I, Boc) | Co(acac)3 | TMEDA | 1a/2a = 21:79 | n. d. |
| 3 | 1a (I, Boc) | Co(acac)3 | TMCD | 1a/2a = 13:87 | n. d. |
| 4 | 1a (I, Boc) | CoCl2 | TMCD | 1a/2a = 0:100 | 2a (81%) |
| 5[d] | 1b (I, Ts) | CoCl2 | TMCD | 1b/2b = 0:100 | 2b (69%) |
| 6 [d], [e] | 1c (I, Bn) | CoCl2 | TMCD | 1c/2c = 0:100 | 2c (66%) |
| 7 [d] | 1d (Br, Boc) | CoCl2 | TMCD | 1d/2a = 0:100 | 2a (83%) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barré, B.; Gonnard, L.; Guérinot, A.; Cossy, J. Cobalt-Catalyzed (Hetero)arylation of Saturated Cyclic Amines with Grignard Reagents. Molecules 2018, 23, 1449. https://doi.org/10.3390/molecules23061449
Barré B, Gonnard L, Guérinot A, Cossy J. Cobalt-Catalyzed (Hetero)arylation of Saturated Cyclic Amines with Grignard Reagents. Molecules. 2018; 23(6):1449. https://doi.org/10.3390/molecules23061449
Chicago/Turabian StyleBarré, Baptiste, Laurine Gonnard, Amandine Guérinot, and Janine Cossy. 2018. "Cobalt-Catalyzed (Hetero)arylation of Saturated Cyclic Amines with Grignard Reagents" Molecules 23, no. 6: 1449. https://doi.org/10.3390/molecules23061449
APA StyleBarré, B., Gonnard, L., Guérinot, A., & Cossy, J. (2018). Cobalt-Catalyzed (Hetero)arylation of Saturated Cyclic Amines with Grignard Reagents. Molecules, 23(6), 1449. https://doi.org/10.3390/molecules23061449