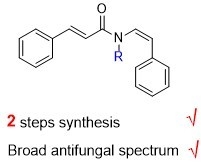
Synthesis and Fungicidal Activity of Lansiumamide A and B and Their Derivatives
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Syntheses
2.2. Fugicidal Activity
3. Experimental Section
3.1. General Information
3.2. Synthetic Procedures
3.2.1. General Synthetic Procedure for Lansiumamide A (1)
3.2.2. General Synthetic Procedure for Lansiumamide B (2) the Title Compounds 3
3.3. Bioassays
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Lin, J.-H. Cinnamamide derivatives from Clausena Lansium. Phytochemistry 1989, 28, 621–622. [Google Scholar] [CrossRef]
- Huang, L.; Feng, Z.; Wang, Y.T.; Lin, L.G. Anticancer carbazole alkaloids and coumarins from Clausena plants: A review. Chin. J. Nat. Med. 2017, 15, 881–888. [Google Scholar] [CrossRef]
- Huang, L.; Li, D.; Xu, Y.S.; Feng, Z.L.; Meng, F.C.; Zhang, Q.W.; Gan, L.S.; Lin, L.G. Clausoxamine, an alkaloid possessing a 1,3-oxazine-4-one ring from the seeds of Clausena lansium and the anti-obesity effect of lansiumamide B. RSC Adv. 2017, 7, 46900–46905. [Google Scholar] [CrossRef]
- Liu, Y.Q.; Staerk, D.; Nielsen, M.N.; Nyberg, N.; Jager, A.K. High-resolution hyaluronidase inhibition profiling combined with HPLC-HRMS-SPE-NMR for identification of anti-necrosis constituents in Chinese plants used to treat snakebite. Phytochemistry 2015, 119, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Ito, C.; Furukawa, H.; Okada, T.; Itoigawa, M. Lansiumamide B and SB-204900 isolated from Clausena lansium inhibit histamine and TNF-alpha release from RBL-2H3 cells. Inflamm. Res. 2013, 62, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.L.; Qin, L.Y.; Wan, S.Q.; Ma, Y.F.; Qin, J.L.; Ma, Y.L. Insecticidal activity and identification of the active ingredients of Clausena lansium seed extracts against larval Spodoptera litura (Lepidoptera: Noctuidae). Acta Entomol. Sin. 2016, 59, 839–845. [Google Scholar]
- Li, L.; Feng, X.; Tang, M.; Hao, W.; Han, Y.; Zhang, G.; Wan, S. Antibacterial activity of Lansiumamide B to tobacco bacterial wilt (Ralstonia solanacearum). Microbiol. Res. 2014, 169, 522–526. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Li, L.; Hao, W.; Tang, M.; Wan, S. Larvicidal activity of lansiumamide B from the seeds of Clausena lansium against Aedes albopictus (Diptera: Culicidae). Parasitol Res. 2013, 112, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Yin, Y.; Guo, Q.; Han, Y.; Wang, L.; Wan, S. Preparation, characterization and nematicidal activity of lansiumamide B nano-capsules. J. Integr. Agric. 2012, 11, 1151–1158. [Google Scholar] [CrossRef]
- Ma, F.; Wan, S.; Liu, X.; Zhao, F. Active ingredients of seed extracts of Clausena lansium and nematiocidal activity against Bursaphelenchus xylophilus. J. South China Agric. Univ. 2009, 1, 23–26. [Google Scholar]
- Shen, L.H.; Jiao, J.; Wang, Y.; Song, Y.Q.; Xu, Y.N.; Sun, H. The extraction and separation of agricultural active constituents in Clausena lansium. Agrochemicals 2015, 54, 39–41. [Google Scholar]
- Liu, Y.; Gong, Z.; Wan, S. Inhibitory effects of clausenamide alkaloid on seven fruit pathogenic fungi. Plant Prot. 2009, 35, 53–56. [Google Scholar]
- Zhang, R.; Wan, S.; Zhao, D. Advances in chemical constituents and biological activities of Clausena lansium. Nat. Prod. Res. Dev. 2012, 24, 118–123. [Google Scholar]
- Stefanuti, I.; Smith, S. A; Taylor, R.J.K. Unsaturated enamides via organometallic addition to isocyanates: the synthesis of Lansamide-I, Lansiumamides A–C and SB-204900. Tetrahedron Lett. 2000, 41, 3735–3738. [Google Scholar] [CrossRef]
- Bayer, A.; Maier, M.E. Synthesis of enamides from aldehydes and amides. Tetrahedron 2004, 60, 6665–6677. [Google Scholar] [CrossRef]
- Fürstner, A.; Brehm, C.; Cancho-Grande, Y. Stereoselective synthesis of enamides by a Peterson reaction manifold. Org. Lett. 2001, 3, 3955–3957. [Google Scholar] [CrossRef] [PubMed]
- Gooβen, L.J.; Blanchot, M.; Arndt, M.; Salih, K.S.M. Synthesis of botryllamides and Lansiumamides via ruthenium-catalyzed hydroamidation of alkynes. Synlett. 2010, 2010, 1685–1687. [Google Scholar]
- Pasqua, A.E.; Ferrari, F.D.; Crawford, J.J.; Marquez, R. Total synthesis of lansiumamides A and B and alatamide. Tetrahedron Lett. 2014, 55, 6042–6043. [Google Scholar] [CrossRef]
- Savary, S.; Teng, P.S.; Willocquet, L.; Nutter, F.W., Jr. Quantification and modeling of croplosses: A review of purposes. Annu. Rev. Phytopathol. 2006, 44, 89–112. [Google Scholar] [CrossRef] [PubMed]
- Delp, C.J. Coping with resistance to plant disease control agents. Plant Dis. 1980, 64, 652–658. [Google Scholar] [CrossRef]
- Cantrell, C.L.; Dayan, F.E.; Duke, S.O. Natural products as sources for new pesticides. J. Nat. Prod. 2012, 75, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wan, S. Antifungal activity and structure-function relationships of synthetic cinnamic amide compounds against Colletotrichum gloeosporioides. J. Zhongkai Univ. Agric. Eng. 2013, 26, 16–21. [Google Scholar]
Sample Availability: Samples of the compounds 1, 2, 3a–i are available from the authors. |


| Compounds | Average Inhibition Rate ± SD (%) (n = 3) | |||||
|---|---|---|---|---|---|---|
| No. | R | Thanatephorus cucumeris | Sclerotinia sclerotiorum | Botrytis cinerea | Phytophthora sojae | Fusahum graminearum |
| 1 | H | 53.89 ± 0.19 | 64.57 ± 0.50 | 27.09 ± 1.08 | 33.08 ± 0.61 | 18.09 ± 0.58 |
| 2 | Me | 91.20 ± 0.87 | 87.52 ± 2.49 | 80.60 ± 0.46 | 79.20 ± 1.76 | 76.63 ± 0.50 |
| 3a | Et | 93.33 ± 0.66 | 66.55 ± 0.85 | 68.08 ± 1.14 | 62.16 ± 0.57 | 49.90 ± 0.55 |
| 3b | n-Pr | 63.94 ± 1.14 | 58.23 ± 1.36 | 37.16 ± 0.96 | 49.41 ± 1.60 | 30.52 ± 1.21 |
| 3c | n-Bu | 42.41 ± 0.95 | 43.61 ± 1.35 | 19.03 ± 0.76 | 36.57 ± 0.78 | 20.83 ± 1.12 |
| 3d | allyl | 70.80 ± 0.94 | 62.45 ± 1.03 | 40.45 ± 0.85 | 60.19 ± 1.48 | 41.67 ± 1.82 |
| 3e | propargyl | 64.28 ± 0.63 | 64.90 ± 1.17 | 77.70 ± 1.43 | 47.34 ± 0.57 | 62.04 ± 0.89 |
| 3f | 2-methylallyl | 47.13 ± 0.49 | 35.96 ± 0.95 | 29.50 ± 0.87 | 53.94 ± 0.78 | 17.08 ± 1.39 |
| 3g | prenyl | 40.02 ± 1.58 | 26.98 ± 1.28 | 32.97 ± 1.98 | 19.47 ± 1.43 | 6.74 ± 0.44 |
| 3h | CH2CO2Et | 27.34 ± 1.25 | 18.02 ± 0.37 | 27.23 ± 0.27 | 21.75 ± 0.62 | 26.95 ± 0.66 |
| 3i | benzyl | 5.07 ± 0.81 | 25.00 ± 1.72 | 49.18 ± 0.93 | 59.01 ± 0.71 | 27.09 ± 0.76 |
| Carbendazim | 100 | 100 | 100 | 100 | 100 | |
| Compounds | Average Inhibition Rate ± SD (%) (n = 3) | |||||
|---|---|---|---|---|---|---|
| No. | R | Rhizoctoniasolani kuehn | Gloeosporium piperatum | Fusarium solani | Alternaria solani | Pyricularia grisea |
| 1 | H | 27.25 ± 0.37 | 18.43 ± 0.94 | 15.94 ± 0.72 | 33.72 ± 0.46 | 17.76 ± 0.83 |
| 2 | Me | 71.23 ± 1.20 | 66.21 ± 0.76 | 63.81 ± 0.23 | 52.52 ± 0.24 | 51.82 ± 1.30 |
| 3a | Et | 49.81 ± 0.34 | 60.40 ± 1.47 | 67.40 ± 0.23 | 60.83 ± 0.35 | 45.32 ± 0.39 |
| 3b | n-Pr | 47.97 ± 1.71 | 46.92 ± 0.56 | 38.86 ± 1.08 | 41.86 ± 0.99 | 43.03 ± 1.05 |
| 3c | n-Bu | 33.99 ± 1.26 | 43.27 ± 1.24 | 33.14 ± 1.71 | 29.99 ± 0.35 | 11.50 ± 0.96 |
| 3d | allyl | 48.98 ± 0.87 | 53.03 ± 0.60 | 52.29 ± 1.71 | 48.89 ± 0.68 | 30.67 ± 2.04 |
| 3e | propargyl | 47.79 ± 0.71 | 65.22 ± 1.46 | 51.51 ± 0.55 | 55.69 ± 0.69 | 47.40 ± 0.29 |
| 3f | 2-methylallyl | 35.17 ± 1.72 | 47.56 ± 0.89 | 30.73 ± 1.05 | 35.06 ± 0.44 | 9.09 ± 1.44 |
| 3g | prenyl | 31.87 ± 1.44 | 13.44 ± 0.27 | 27.30 ± 0.65 | 23.89 ± 1.26 | 15.50 ± 0.91 |
| 3h | CH2CO2Et | 31.31 ± 2.02 | 25.38 ± 2.01 | 26.44 ± 0.55 | 45.50 ± 1.53 | 10.59 ± 0.97 |
| 3i | benzyl | 20.82 ± 1.07 | 48.30 ± 1.21 | 19.37 ± 0.34 | 27.31 ± 1.15 | 32.94 ± 0.70 |
| Carbendazim | 100 | 100 | 100 | 1.25 ± 0.37 | 100 | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, H.; Chen, T.; Huang, L.; Shen, Q.; Lian, Z.; Shi, Y.; Ouyang, M.-A.; Song, L. Synthesis and Fungicidal Activity of Lansiumamide A and B and Their Derivatives. Molecules 2018, 23, 1499. https://doi.org/10.3390/molecules23071499
Xu H, Chen T, Huang L, Shen Q, Lian Z, Shi Y, Ouyang M-A, Song L. Synthesis and Fungicidal Activity of Lansiumamide A and B and Their Derivatives. Molecules. 2018; 23(7):1499. https://doi.org/10.3390/molecules23071499
Chicago/Turabian StyleXu, Huiyou, Ting Chen, Luanbin Huang, Qiuju Shen, Zengwei Lian, Yan Shi, Ming-An Ouyang, and Liyan Song. 2018. "Synthesis and Fungicidal Activity of Lansiumamide A and B and Their Derivatives" Molecules 23, no. 7: 1499. https://doi.org/10.3390/molecules23071499
APA StyleXu, H., Chen, T., Huang, L., Shen, Q., Lian, Z., Shi, Y., Ouyang, M. -A., & Song, L. (2018). Synthesis and Fungicidal Activity of Lansiumamide A and B and Their Derivatives. Molecules, 23(7), 1499. https://doi.org/10.3390/molecules23071499