Recent Advances in Toll Like Receptor-Targeting Glycoconjugate Vaccines
Abstract
:1. Introduction
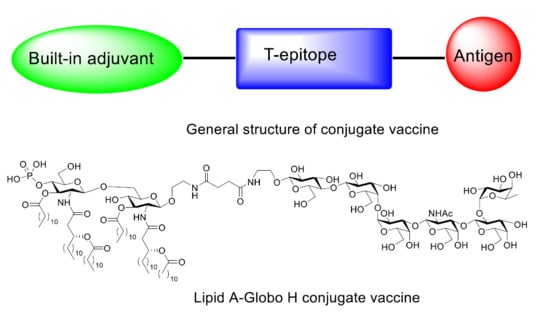
1.1. Conjugate Vaccines
1.2. Toll Like Receptors
2. Lipopeptides as Carrier Molecules or Adjuvants for Vaccine Development
3. MPLAs as Carrier Molecules or Adjuvants for Vaccine Development
3.1. TLR4 Ligands as Adjuvants
3.2. Use of Lipid A Derivatives for Vaccine Development and/or as External Adjuvants
3.3. MPLA Analogs as Carrier Molecules and Built-in Adjuvants for Conjugate Vaccine Development
3.4. Conjugate Vaccines with MPLA Mimics as Carrier Molecules or Built-In Adjuvants
4. Other TLR Ligand Conjugates
5. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Fuster, M.M.; Esko, J.D. The sweet and sour of cancer: Glycans as novel therapeutic targets. Nat. Rev. Cancer 2005, 5, 526–542. [Google Scholar] [CrossRef] [PubMed]
- Ohtsubo, K.; Marth, J.D. Glycosylation in cellular mechanisms of health and disease. Cell 2006, 126, 855–867. [Google Scholar] [CrossRef] [PubMed]
- Stowell, S.R.; Ju, T.; Cummings, R.D. Protein Glycosylation in Cancer. Annu. Rev. Pathol. Mech. Dis. 2015, 10, 473–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kayhty, H.; Karanko, V.; Peltola, H.; Makela, P.H. Serum antibodies after vaccination with Haemophilus influenzae type b capsular polysaccharide and responses to reimmunization: No evidence of immunologic tolerance or memory. Pediatrics 1984, 74, 857–865. [Google Scholar] [PubMed]
- O’Brien, K.L.; Steinhoff, M.C.; Edwards, K.; Keyserling, H.; Thoms, M.L.; Madore, D. Immunologic priming of young children by pneumococcal glycoprotein conjugate, but not polysaccharide, vaccines. Pediatr. Infect. Dis. J. 1996, 15, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Guttormsen, H.-K.; Sharpe, A.H.; Chandraker, A.K.; Brigtsen, A.K.; Sayegh, M.H.; Kasper, D.L. Cognate stimulatory B-cell-T-cell interactions are critical for T-cell help recruited by glycoconjugate vaccines. Infect. Immun. 1999, 67, 6375–6384. [Google Scholar] [PubMed]
- Goldblatt, D. Conjugate vaccines. Clin. Exp. Immunol. 2000, 119, 1–3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gold, R.; Lepow, M.L.; Goldschneider, I.; Draper, T.L.; Gotschlich, E.C. Clinical evaluation of group A and group C meningococcal polysaccharide vaccines in infants. J. Clin. Investig. 1975, 56, 1536–1547. [Google Scholar] [CrossRef] [PubMed]
- Coffman, R.L.; Sher, A.; Seder, R.A. Vaccine Adjuvants: Putting Innate Immunity to Work. Immunity 2010, 33, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Awate, S.; Babiuk, L.A.; Mutwiri, G. Mechanisms of action of adjuvants. Front. Immunol. 2013, 4, 114. [Google Scholar] [CrossRef] [PubMed]
- Avci, F.Y.; Kasper, D.L. How bacterial carbohydrates influence the adaptive immune system. Annu. Rev. Immunol. 2010, 28, 107–130. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Irvine, D.J. Guiding Principles in the Design of Molecular Bioconjugates for Vaccine Applications. Bioconjug. Chem. 2015, 26, 791–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durando, P.; Faust Saul, N.; Torres, A. Immunological Features and Clinical Benefits of Conjugate Vaccines against Bacteria. J. Immunol. Res. 2015, 2015, 934504. [Google Scholar] [CrossRef] [PubMed]
- Goebel, W.F.; Avery, O.T. Chemo-immunological studies on conjugated carbohydrate-proteins. I. The synthesis of p-aminophenol β-glucoside, p-aminophenol β-galactoside, and their coupling with serum globulin. J. Exp. Med. 1929, 50, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Tillett, W.S.; Avery, O.T.; Goebel, W.F. Chemo-immunological studies on conjugated carbohydrate-proteins. III. Active and passive anaphylaxis with synthetic sugar-proteins. J. Exp. Med. 1929, 50, 551–567. [Google Scholar] [CrossRef] [PubMed]
- Avery, O.T.; Goebel, W.F. Chemo-immunological studies on conjugated carbohydrate-proteins. II. Immunological specificity of synthetic sugar-protein antigens. J. Exp. Med. 1929, 50, 533–550. [Google Scholar] [CrossRef] [PubMed]
- Pichichero, M.E. Protein carriers of conjugate vaccines: Characteristics, development, and clinical trials. Hum. Vaccines Immunother. 2013, 9, 2505–2523. [Google Scholar] [CrossRef] [PubMed]
- Micoli, F.; Costantino, P.; Adamo, R. Potential targets for next generation antimicrobial glycoconjugate vaccines. FEMS Microbiol. Rev. 2018, 42, 388–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Astronomo, R.D.; Burton, D.R. Carbohydrate vaccines: Developing sweet solutions to sticky situations? Nat. Rev. Drug Discov. 2010, 9, 308–324. [Google Scholar] [CrossRef] [PubMed]
- Avci, F.Y.; Li, X.; Tsuji, M.; Kasper, D.L. A mechanism for glycoconjugate vaccine activation of the adaptive immune system and its implications for vaccine design. Nat. Med. 2011, 17, 1602–1609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwasaki, A.; Medzhitov, R. Toll-like receptor control of the adaptive immune responses. Nat. Immunol. 2004, 5, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Takeda, K.; Kaisho, T. Toll-like receptors: Critical proteins linking innate and acquired immunity. Nat. Immunol. 2001, 2, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Kaufmann, A.; Grote, K.; Kawai, T.; Hoshino, K.; Morr, M.; Muhlradt, P.F.; Akira, S. Cutting edge: Preferentially the R-stereoisomer of the mycoplasmal lipopeptide macrophage-activating lipopeptide-2 activates immune cells through a toll-like receptor 2- and MyD88-dependent signaling pathway. J. Immunol. 2000, 164, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Takeuchi, O.; Fujita, T.; Inoue, J.-I.; Muhlradt, P.F.; Sato, S.; Hoshino, K.; Akira, S. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J. Immunol. 2001, 167, 5887–5894. [Google Scholar] [CrossRef] [PubMed]
- Park, B.S.; Song, D.H.; Kim, H.M.; Choi, B.-S.; Lee, H.; Lee, J.-O. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 2009, 458, 1191–1195. [Google Scholar] [CrossRef] [PubMed]
- Ignacio, B.J.; Albin, T.J.; Esser-Kahn, A.P.; Verdoes, M. Toll-like Receptor Agonist Conjugation: A Chemical Perspective. Bioconjug. Chem. 2018, 29, 587–603. [Google Scholar] [CrossRef] [PubMed]
- Hess, N.J.; Felicelli, C.; Grage, J.; Tapping, R.I. TLR10 suppresses the activation and differentiation of monocytes with effects on DC-mediated adaptive immune responses. J. Leukoc. Biol. 2017, 101, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Fujita, Y.; Taguchi, H. Overview and outlook of Toll-like receptor ligand-antigen conjugate vaccines. Ther. Deliv. 2012, 3, 749–760. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Sato, S.; Horiuchi, T.; Hoshino, K.; Takeda, K.; Dong, Z.; Modlin, R.L.; Akira, S. Cutting edge: Role of toll-like receptor 1 in mediating immune response to microbial lipoproteins. J. Immunol. 2002, 169, 10–14. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.C.; Lau, Y.F.; Le, T.; Suhrbier, A.; Deliyannis, G.; Cheers, C.; Smith, C.; Zeng, W.; Brown, L.E. A totally synthetic vaccine of generic structure that targets toll-like receptor 2 on dendritic cells and promotes antibody or cytotoxic T cell responses. Proc. Natl. Acad. Sci. USA 2004, 101, 15440–15445. [Google Scholar] [CrossRef] [PubMed]
- Hopp, T.P. Immunogenicity of a synthetic HBsAg peptide: Enhancement by conjugation to a fatty acid carrier. Mol. Immunol. 1984, 21, 13–16. [Google Scholar] [CrossRef]
- Jung, G.; Wiesmueller, K.H.; Becker, G.; Buhring, H.J.; Bessler, W.G. Increased production of specific antibodies by presentation of antigen determinants with covalently bound lipopeptide mitogens. Angew. Chem. 1985, 97, 883–885. [Google Scholar] [CrossRef]
- Toyokuni, T.; Hakomori, S.; Singhal, A.K. Synthetic carbohydrate vaccines: Synthesis and immunogenicity of Tn antigen conjugates. Bioorg. Med. Chem. 1994, 2, 1119–1132. [Google Scholar] [CrossRef]
- Toyokuni, T.; Dean, B.; Cai, S.; Boivin, D.; Hakomori, S.; Singhal, A.K. Synthetic vaccines: Synthesis of a dimeric Tn antigen-lipopeptide conjugate that elicits immune responses against Tn-expressing glycoproteins. J. Am. Chem. Soc. 1994, 116, 395–396. [Google Scholar] [CrossRef]
- Kudryashov, V.; Glunz, P.W.; Williams, L.J.; Hintermann, S.; Danishefsky, S.J.; Lloyd, K.O. Toward optimized carbohydrate-based anticancer vaccines: Epitope clustering, carrier structure, and adjuvant all influence antibody responses to Lewisy conjugates in mice. Proc. Natl. Acad. Sci. USA 2001, 98, 3264–3269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Glunz, P.W.; Hintermann, S.; Schwarz, J.B.; Kuduk, S.D.; Chen, X.-T.; Williams, L.J.; Sames, D.; Danishefsky, S.J.; Kudryashov, V.; Lloyd, K.O. Probing cell surface “Glyco-Architecture” through total synthesis. Immunological consequences of a human blood group determinant in a clustered mucin-like context. J. Am. Chem. Soc. 1999, 121, 10636–10637. [Google Scholar] [CrossRef]
- Keding, S.J.; Danishefsky, S.J. Prospects for total synthesis: A vision for a totally synthetic vaccine targeting epithelial tumors. Proc. Natl. Acad. Sci. USA 2004, 101, 11937–11942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buskas, T.; Ingale, S.; Boons, G.-J. Towards a fully synthetic carbohydrate-based anticancer vaccine: Synthesis and immunological evaluation of a lipidated glycopeptide containing the tumor-associated Tn antigen. Angew. Chem. Int. Ed. 2005, 44, 5985–5988. [Google Scholar] [CrossRef] [PubMed]
- Reichel, F.; Ashton, P.R. Synthetic carbohydrate-based vaccines: Synthesis of an l-glycero-d-manno-heptose antigen-T-epitope-lipopeptide conjugate. Chem. Commun. 1997, 2087–2088. [Google Scholar] [CrossRef]
- Nalla, N.; Pallavi, P.; Reddy, B.S.; Miryala, S.; Naveen Kumar, V.; Mahboob, M.; Halmuthur, M.S.K. Design, synthesis and immunological evaluation of 1,2,3-triazole-tethered carbohydrate-Pam3Cys conjugates as TLR2 agonists. Bioorg. Med. Chem. 2015, 23, 5846–5855. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Huang, Z.-H.; Shi, L.; Zhao, Y.-F.; Kunz, H.; Li, Y.-M. Towards a Fully Synthetic MUC1-Based Anticancer Vaccine: Efficient Conjugation of Glycopeptides with Mono-, Di-, and Tetravalent Lipopeptides Using Click Chemistry. Chem. Eur. J. 2011, 17, 6396–6406. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, B.L.; Malins, L.R.; Chun, C.K.Y.; Payne, R.J. Synthesis of MUC1-lipopeptide chimeras. Chem. Commun. 2010, 46, 6249–6251. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.K.; Vartak, A.; Karmakar, P.; Sucheck, S.J.; Wall, K.A. Augmenting vaccine immunogenicity through the use of natural human anti-rhamnose antibodies. ACS Chem. Biol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Salyer, A.C.D.; Wall, K.A.; Sucheck, S.J. Synthesis and Immunological Evaluation of a MUC1 Glycopeptide Incorporated into l-Rhamnose Displaying Liposomes. Bioconjug. Chem. 2013, 24, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, P.; Lee, K.; Sarkar, S.; Wall, K.A.; Sucheck, S.J. Synthesis of a Liposomal MUC1 Glycopeptide-Based Immunotherapeutic and Evaluation of the Effect of l-Rhamnose Targeting on Cellular Immune Responses. Bioconjug. Chem. 2016, 27, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Ingale, S.; Wolfert, M.A.; Gaekwad, J.; Buskas, T.; Boons, G.-J. Robust immune responses elicited by a fully synthetic three-component vaccine. Nat. Chem. Biol. 2007, 3, 663–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakshminarayanan, V.; Thompson, P.; Wolfert, M.A.; Buskas, T.; Bradley, J.M.; Pathangey, L.B.; Madsen, C.S.; Cohen, P.A.; Gendler, S.J.; Boons, G.-J. Immune recognition of tumor-associated mucin MUC1 is achieved by a fully synthetic aberrantly glycosylated MUC1 tripartite vaccine. Proc. Natl. Acad. Sci. USA 2012, 109, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Ingale, S.; Buskas, T.; Boons, G.-J. Synthesis of Glyco(lipo)peptides by Liposome-Mediated Native Chemical Ligation. Org. Lett. 2006, 8, 5785–5788. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Cai, H.; Huang, Z.-H.; Sun, Z.-Y.; Chen, Y.-X.; Zhao, Y.-F.; Kunz, H.; Li, Y.-M. Synthetic MUC1 Antitumor Vaccine Candidates with Varied Glycosylation Pattern Bearing R/S-configured Pam3CysSerLys4. ChemBioChem 2016, 17, 1412–1415. [Google Scholar] [CrossRef] [PubMed]
- Bessler, W.G.; Cox, M.; Lex, A.; Suhr, B.; Wiesmueller, K.H.; Jung, G. Synthetic lipopeptide analogs of bacterial lipoprotein are potent polyclonal activators for murine B lymphocytes. J. Immunol. 1985, 135, 1900–1905. [Google Scholar] [PubMed]
- Cai, H.; Sun, Z.-Y.; Chen, M.-S.; Zhao, Y.-F.; Kunz, H.; Li, Y.-M. Synthetic multivalent glycopeptide-lipopeptide antitumor vaccines: Impact of the cluster effect on the killing of tumor cells. Angew. Chem. Int. Ed. 2014, 53, 1699–1703. [Google Scholar] [CrossRef] [PubMed]
- Renaudet, O.; Dasgupta, G.; Bettahi, I.; Shi, A.; Nesburn, A.B.; Dumy, P.; BenMohamed, L. Linear and branched glyco-lipopeptide vaccines follow distinct cross-presentation pathways and generate different magnitudes of antitumor immunity. PLoS ONE 2010, 5, e11216. [Google Scholar] [CrossRef] [PubMed]
- Grigalevicius, S.; Chierici, S.; Renaudet, O.; Lo-Man, R.; Deriaud, E.; Leclerc, C.; Dumy, P. Chemoselective Assembly and Immunological Evaluation of Multiepitopic Glycoconjugates Bearing Clustered Tn Antigen as Synthetic Anticancer Vaccines. Bioconjug. Chem. 2005, 16, 1149–1159. [Google Scholar] [CrossRef] [PubMed]
- Dumy, P.; Eggleston, I.M.; Cervigni, S.; Sila, U.; Sun, X.; Mutter, M. A convenient synthesis of cyclic peptides as regioselectively addressable functionalized templates (RAFT). Tetrahedron Lett. 1995, 36, 1255–1258. [Google Scholar] [CrossRef]
- Hewitt, M.C.; Seeberger, P.H. Solution and Solid-Support Synthesis of a Potential Leishmaniasis Carbohydrate Vaccine. J. Org. Chem. 2001, 66, 4233–4243. [Google Scholar] [CrossRef] [PubMed]
- Muhlradt, P.F.; Kiess, M.; Meyer, H.; Sussmuth, R.; Jung, G. Structure and specific activity of macrophage-stimulating lipopeptides from Mycoplasma hyorhinis. Infect. Immun. 1998, 66, 4804–4810. [Google Scholar] [PubMed]
- Huynh, A.S.; Chung, W.J.; Cho, H.-I.; Moberg, V.E.; Celis, E.; Morse, D.L.; Vagner, J. Novel Toll-like Receptor 2 Ligands for Targeted Pancreatic Cancer Imaging and Immunotherapy. J. Med. Chem. 2012, 55, 9751–9762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, D.M.; Wilkinson, B.L.; Corcilius, L.; Thaysen-Andersen, M.; Byrne, S.N.; Payne, R.J. Synthesis and immunological evaluation of self-adjuvanting MUC1-macrophage activating lipopeptide 2 conjugate vaccine candidates. Chem. Commun. 2014, 50, 10273–10276. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, B.L.; Day, S.; Malins, L.R.; Apostolopoulos, V.; Payne, R.J. Self-Adjuvanting Multicomponent Cancer Vaccine Candidates Combining Per-Glycosylated MUC1 Glycopeptides and the Toll-like Receptor 2 Agonist Pam3CysSer. Angew. Chem. Int. Ed. 2011, 50, 1635–1639. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, B.L.; Day, S.; Chapman, R.; Perrier, S.; Apostolopoulos, V.; Payne, R.J. Synthesis and Immunological Evaluation of Self-Assembling and Self-Adjuvanting Tricomponent Glycopeptide Cancer-Vaccine Candidates. Chem. Eur. J. 2012, 18, 16540–16548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Ramos, T.V.; Gras-Masse, H.; Kaplan, B.E.; BenMohamed, L. Lipopeptide epitopes extended by an Nε-palmitoyl-lysine moiety increase uptake and maturation of dendritic cells through a Toll-like receptor-2 pathway and trigger a Th1-dependent protective immunity. Eur. J. Immunol. 2004, 34, 3102–3114. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, A.; Gaidzik, N.; Becker, T.; Menge, C.; Groh, K.; Cai, H.; Li, Y.-M.; Gerlitzki, B.; Schmitt, E.; Kunz, H. Fully Synthetic Vaccines Consisting of Tumor-Associated MUC1 Glycopeptides and a Lipopeptide Ligand of the Toll-like Receptor 2. Angew. Chem. Int. Ed. 2010, 49, 3688–3692. [Google Scholar] [CrossRef] [PubMed]
- Dziadek, S.; Griesinger, C.; Kunz, H.; Reinscheid, U.M. Synthesis and structural model of an α(2,6)-sialyl-T glycosylated MUC1 eicosapeptide under physiological conditions. Chem. Eur. J. 2006, 12, 4981–4993. [Google Scholar] [CrossRef] [PubMed]
- Fontenot, J.D.; Tjandra, N.; Bu, D.; Ho, C.; Montelaro, R.C.; Finn, O.J. Biophysical characterization of one-, two-, and three-tandem repeats of human mucin (muc-1) protein core. Cancer Res. 1993, 53, 5386–5394. [Google Scholar] [PubMed]
- Mollick Joseph, A.; Hodi, F.S.; Soiffer Robert, J.; Nadler Lee, M.; Dranoff, G. MUC1-like tandem repeat proteins are broadly immunogenic in cancer patients. Cancer Immun. 2003, 3, 3. [Google Scholar]
- Jin, M.S.; Kim, S.E.; Heo, J.Y.; Lee, M.E.; Kim, H.M.; Paik, S.-G.; Lee, H.; Lee, J.-O. Crystal structure of the TLR1-TLR2 heterodimer induced by binding of a tri-acylated lipopeptide. Cell 2007, 130, 1071–1082. [Google Scholar] [CrossRef] [PubMed]
- Seyberth, T.; Voss, S.; Brock, R.; Wiesmueller, K.-H.; Jung, G. Lipolanthionine Peptides Act as Inhibitors of TLR2-Mediated IL-8 Secretion. Synthesis and Structure-Activity Relationships. J. Med. Chem. 2006, 49, 1754–1765. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Li, R.; Malladi, S.S.; Warshakoon, H.J.; Kimbrell, M.R.; Amolins, M.W.; Ukani, R.; Datta, A.; David, S.A. Structure-Activity Relationships in Toll-like Receptor-2 Agonistic Diacylthioglycerol Lipopeptides. J. Med. Chem. 2010, 53, 3198–3213. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.-G.; Eriksson, E.; Chua, B.; Grollo, L.; Jackson, D.C. Structural requirement for the agonist activity of the TLR2 ligand Pam2Cys. Amino Acids 2010, 39, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Willems, M.M.J.H.P.; Zom, G.G.; Khan, S.; Meeuwenoord, N.; Melief, C.J.M.; van der Stelt, M.; Overkleeft, H.S.; Codee, J.D.C.; van der Marel, G.A.; Ossendorp, F.; et al. N-Tetradecylcarbamyl Lipopeptides as Novel Agonists for Toll-like Receptor 2. J. Med. Chem. 2014, 57, 6873–6878. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wu, N.; Liu, X.; Wu, T.; Zhou, Y.; Huang, J.; Wu, L.; Shang, Y.; Liu, X.; Liao, X. The Novel Toll-Like Receptor 2 Agonist SUP3 Enhances Antigen Presentation and T Cell Activation by Dendritic Cells. Front. Immunol. 2017, 8, 158. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Ghosh, S.; Lau Yuk, F.; Brown Lorena, E.; Jackson David, C. Highly immunogenic and totally synthetic lipopeptides as self-adjuvanting immunocontraceptive vaccines. J. Immunol. 2002, 169, 4905–4912. [Google Scholar] [CrossRef] [PubMed]
- Zaman, M.; Abdel-Aal, A.-B.M.; Phillipps, K.S.M.; Fujita, Y.; Good, M.F.; Toth, I. Structure-activity relationship of lipopeptide Group A streptococcus (GAS) vaccine candidates on toll-like receptor 2. Vaccine 2010, 28, 2243–2248. [Google Scholar] [CrossRef] [PubMed]
- McDonald, D.M.; Byrne, S.N.; Payne, R.J. Synthetic self-adjuvanting glycopeptide cancer vaccines. Front. Chem. 2015, 3, 60. [Google Scholar] [CrossRef] [PubMed]
- Buskas, T.; Thompson, P.; Boons, G.-J. Immunotherapy for cancer: Synthetic carbohydrate-based vaccines. Chem. Commun. 2009, 5335–5349. [Google Scholar] [CrossRef] [PubMed]
- Poltorak, A.; He, X.; Smirnova, I.; Liu, M.-Y.; Van Huffel, C.; Du, X.; Birdwell, D.; Alejos, E.; Silva, M.; Galanos, C.; et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: Mutations in Tlr4 gene. Science 1998, 282, 2085–2088. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Takeda, K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004, 4, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Wu, Y.; Huang, X.; Wang, W.; Bing, A.; Cao, X.; Wan, T. Toll-like Receptor 4 (TLR4) Is Essential for Hsp70-like Protein 1 (HSP70L1) to Activate Dendritic Cells and Induce Th1 Response. J. Biol. Chem. 2011, 286, 30393–30400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hutchinson, M.R.; Zhang, Y.; Shridhar, M.; Evans, J.H.; Buchanan, M.M.; Zhao, T.X.; Slivka, P.F.; Coats, B.D.; Rezvani, N.; Wieseler, J.; et al. Evidence that opioids may have toll-like receptor 4 and MD-2 effects. Brain Behav. Immun. 2010, 24, 83–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peana, M.; Zdyb, K.; Medici, S.; Pelucelli, A.; Simula, G.; Gumienna-Kontecka, E.; Zoroddu, M.A. Ni(II) interaction with a peptide model of the human TLR4 ectodomain. J. Trace Elem. Med. Biol. 2017, 44, 151–160. [Google Scholar] [CrossRef] [PubMed]
- Qureshi, N.; Takayama, K.; Ribi, E. Purification and structural determination of nontoxic lipid A obtained from the lipopolysaccharide of Salmonella typhimurium. J. Biol. Chem. 1982, 257, 11808–11815. [Google Scholar] [PubMed]
- Boland, G.; Beran, J.; Lievens, M.; Sasadeusz, J.; Dentico, P.; Nothdurft, H.; Zuckerman, J.N.; Genton, B.; Steffen, R.; Loutan, L.; et al. Safety and immunogenicity profile of an experimental hepatitis B vaccine adjuvanted with AS04. Vaccine 2004, 23, 316–320. [Google Scholar] [CrossRef] [PubMed]
- Goepfert, P.A.; Tomaras, G.D.; Horton, H.; Montefiori, D.; Ferrari, G.; Deers, M.; Voss, G.; Koutsoukos, M.; Pedneault, L.; Vandepapeliere, P.; et al. Durable HIV-1 antibody and T-cell responses elicited by an adjuvanted multi-protein recombinant vaccine in uninfected human volunteers. Vaccine 2007, 25, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Bojang, K.A.; Milligan, P.J.M.; Pinder, M.; Vigneron, L.; Alloueche, A.; Kester, K.E.; Ballou, W.R.; Conway, D.J.; Reece, W.H.H.; Gothard, P.; et al. Efficacy of RTS,S/AS02 malaria vaccine against Plasmodium falciparum infection in semi-immune adult men in Gambia: A randomized trial. Lancet 2001, 358, 1927–1934. [Google Scholar] [CrossRef]
- Elliott, G.T.; McLeod, R.A.; Perez, J.; Von Eschen, K.B. Interim results of a phase II multicenter clinical trial evaluating the activity of a therapeutic allogeneic melanoma vaccine (theraccine) in the treatment of disseminated malignant melanoma. Semin. Surg. Oncol. 1993, 9, 264–272. [Google Scholar] [PubMed]
- Sosman, J.A.; Unger, J.M.; Liu, P.Y.; Flaherty, L.E.; Park, M.S.; Kempf, R.A.; Thompson, J.A.; Terasaki, P.I.; Sondak, V.K. Adjuvant immunotherapy of resected, intermediate-thickness, node-negative melanoma with an allogeneic tumor vaccine: Impact of HLA class I antigen expression on outcome. J. Clin. Oncol. 2002, 20, 2067–2075. [Google Scholar] [CrossRef] [PubMed]
- Miles, D.W.; Towlson, K.E.; Graham, R.; Reddish, M.; Longenecker, B.M.; Taylor-Papadimitriou, J.; Rubens, R.D. A randomized phase II study of sialyl-Tn and DETOX-B adjuvant with or without cyclophosphamide pretreatment for the active specific immunotherapy of breast cancer. Br. J. Cancer 1996, 74, 1292–1296. [Google Scholar] [CrossRef] [PubMed]
- Butts, C.; Murray, N.; Maksymiuk, A.; Goss, G.; Marshall, E.; Soulieres, D.; Cormier, Y.; Ellis, P.; Price, A.; Sawhney, R.; et al. Randomized phase IIB trial of BLP25 liposome vaccine in stage IIIB and IV non-small-cell lung cancer. J. Clin. Oncol. 2005, 23, 6674–6681. [Google Scholar] [CrossRef] [PubMed]
- North, S.A.; Graham, K.; Bodnar, D.; Venner, P. A pilot study of the liposomal MUC1 vaccine BLP25 in prostate specific antigen failures after radical prostatectomy. J. Urol. 2006, 176, 91–95. [Google Scholar] [CrossRef]
- Persing, D.H.; Coler, R.N.; Lacy, M.J.; Johnson, D.A.; Baldridge, J.R.; Hershberg, R.M.; Reed, S.G. Taking toll: Lipid A mimetics as adjuvants and immunomodulators. Trends Microbiol. 2002, 10 (Suppl. 10), s32–s37. [Google Scholar] [CrossRef]
- Dupont, J.; Altclas, J.; Lepetic, A.; Lombardo, M.; Vazquez, V.; Salgueira, C.; Seigelchifer, M.; Arndtz, N.; Antunez, E.; von Eschen, K.; et al. A controlled clinical trial comparing the safety and immunogenicity of a new adjuvanted hepatitis B vaccine with a standard hepatitis B vaccine. Vaccine 2006, 24, 7167–7174. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Guo, Z. Progress in the synthesis and biological evaluation of lipid A and its derivatives. Med. Res. Rev. 2018, 38, 556–601. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Xue, J.; Guo, Z. Synthesis of a monophosphoryl lipid A derivative and its conjugation to a modified form of a tumor-associated carbohydrate antigen GM3. Chem. Commun. 2009, 5536–5537. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhou, Z.; Tang, S.; Guo, Z. Carbohydrate-Monophosphoryl Lipid A Conjugates Are Fully Synthetic Self-Adjuvanting Cancer Vaccines Eliciting Robust Immune Responses in the Mouse. ACS Chem. Biol. 2012, 7, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Mondal, M.; Liao, G.; Guo, Z. Synthesis and evaluation of monophosphoryl lipid A derivatives as fully synthetic self-adjuvanting glycoconjugate cancer vaccine carriers. Org. Biomol. Chem. 2014, 12, 3238–3245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, S.; Wang, Q.; Guo, Z. Synthesis of a Monophosphoryl Derivative of Escherichia coli Lipid A and Its Efficient Coupling to a Tumor-Associated Carbohydrate Antigen. Chem. Eur. J. 2010, 16, 1319–1325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Danishefsky, S.J.; Shue, Y.-K.; Chang, M.N.; Wong, C.-H. Development of Globo-H cancer vaccine. Acc. Chem. Res. 2015, 48, 643–652. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Liao, G.; Mandal, S.S.; Suryawanshi, S.; Guo, Z. A fully synthetic self-adjuvanting globo H-Based vaccine elicited strong T cell-mediated antitumor immunity. Chem. Sci. 2015, 6, 7112–7121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cluff Christopher, W. Monophosphoryl lipid A (MPL) as an adjuvant for anti-cancer vaccines: Clinical results. Adv. Exp. Med. Biol. 2010, 667, 111–123. [Google Scholar]
- Liao, G.; Zhou, Z.; Suryawanshi, S.; Mondal, M.A.; Guo, Z. Fully Synthetic Self-Adjuvanting α-2,9-Oligosialic Acid Based Conjugate Vaccines against Group C Meningitis. ACS Cent. Sci. 2016, 2, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Feng, S.; Wang, S.; Li, H.; Gu, G.; Guo, Z. Synthesis and Immunological Comparison of Differently Linked Lipoarabinomannan Oligosaccharide-Monophosphoryl Lipid A Conjugates as Antituberculosis Vaccines. J. Org. Chem. 2017, 82, 12085–12096. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Mandal Satadru, S.; Liao, G.; Guo, J.; Guo, Z.; Liao, G.; Guo, J.; Guo, Z. Synthesis and Evaluation of GM2-Monophosphoryl Lipid A Conjugate as a Fully Synthetic Self-Adjuvant Cancer Vaccine. Sci. Rep. 2017, 7, 11403. [Google Scholar] [CrossRef] [PubMed]
- D’Alonzo, D.; Cipolletti, M.; Tarantino, G.; Ziaco, M.; Pieretti, G.; Iadonisi, A.; Palumbo, G.; Alfano, A.; Giuliano, M.; De Rosa, M.; et al. A Semisynthetic Approach to New Immunoadjuvant Candidates: Site-Selective Chemical Manipulation of Escherichia coli Monophosphoryl Lipid A. Chem. Eur. J. 2016, 22, 11053–11063. [Google Scholar] [CrossRef] [PubMed]
- Ziaco, M.; Gorska, S.; Traboni, S.; Razim, A.; Casillo, A.; Iadonisi, A.; Gamian, A.; Corsaro, M.M.; Bedini, E. Development of Clickable Monophosphoryl Lipid A Derivatives toward Semisynthetic Conjugates with Tumor-Associated Carbohydrate Antigens. J. Med. Chem. 2017, 60, 9757–9768. [Google Scholar] [CrossRef] [PubMed]
- Miyajima, K.; Nekado, T.; Ikeda, K.; Achiwa, K. Synthesis of Tn and sialyl Tn antigen-lipid A analog conjugates for synthetic vaccines. Chem. Pharm. Bull. 1997, 45, 1544–1546. [Google Scholar] [CrossRef] [PubMed]
- Miyajima, K.; Gomi, N.; Ikeda, K.; Achiwa, K. Lipid A and related compounds. XXXIII. Synthesis and structure-activity relationships of N-acylated l-serine or l-threonine-containing d-glucosamine derivatives as mimics of lipid A disaccharide. Chem. Pharm. Bull. 1997, 45, 1089–1093. [Google Scholar] [CrossRef]
- Lewicky, J.D.; Ulanova, M.; Jiang, Z.-H. Synthesis and immunostimulatory activity of diethanolamine-containing lipid A mimics. RSC Adv. 2012, 2, 1917–1926. [Google Scholar] [CrossRef]
- Lewicky, J.D.; Ulanova, M.; Jiang, Z.-H. Synthesis of a TLR4 Agonist-Carbohydrate Antigen Conjugate As A Self-Adjuvanting Cancer Vaccine. ChemistrySelect 2016, 1, 906–910. [Google Scholar] [CrossRef]
- Glaffig, M.; Stergiou, N.; Schmitt, E.; Kunz, H. Immunogenicity of a fully synthetic MUC1 glycopeptide antitumor vaccine enhanced by poly(I:C) as a TLR3-activating adjuvant. ChemMedChem 2017, 12, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, A.-B.M.; Lakshminarayanan, V.; Thompson, P.; Supekar, N.; Bradley, J.M.; Wolfert, M.A.; Cohen, P.A.; Gendler, S.J.; Boons, G.-J. Immune and Anticancer Responses Elicited by Fully Synthetic Aberrantly Glycosylated MUC1 Tripartite Vaccines Modified by a TLR2 or TLR9 Agonist. ChemBioChem 2014, 15, 1508–1513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartmann, S.; Nuhn, L.; Palitzsch, B.; Glaffig, M.; Stergiou, N.; Gerlitzki, B.; Schmitt, E.; Kunz, H.; Zentel, R. CpG-Loaded Multifunctional Cationic Nanohydrogel Particles as Self-Adjuvanting Glycopeptide Antitumor Vaccines. Adv. Healthc. Mater. 2015, 4, 522–527. [Google Scholar] [CrossRef] [PubMed]




















© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Guo, Z. Recent Advances in Toll Like Receptor-Targeting Glycoconjugate Vaccines. Molecules 2018, 23, 1583. https://doi.org/10.3390/molecules23071583
Li Q, Guo Z. Recent Advances in Toll Like Receptor-Targeting Glycoconjugate Vaccines. Molecules. 2018; 23(7):1583. https://doi.org/10.3390/molecules23071583
Chicago/Turabian StyleLi, Qingjiang, and Zhongwu Guo. 2018. "Recent Advances in Toll Like Receptor-Targeting Glycoconjugate Vaccines" Molecules 23, no. 7: 1583. https://doi.org/10.3390/molecules23071583
APA StyleLi, Q., & Guo, Z. (2018). Recent Advances in Toll Like Receptor-Targeting Glycoconjugate Vaccines. Molecules, 23(7), 1583. https://doi.org/10.3390/molecules23071583