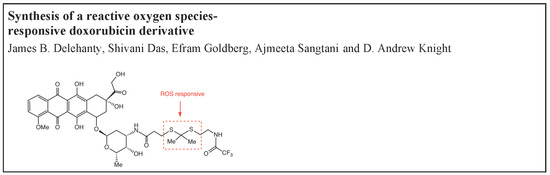
Synthesis of a Reactive Oxygen Species-Responsive Doxorubicin Derivative
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Swain, S.; Sahu, P.K.; Beg, S.; Babu, S.M. Nanoparticles for cancer targeting: Current and future directions. Curr. Drug Deliv. 2016, 13, 1290–1302. [Google Scholar] [CrossRef] [PubMed]
- Sangtani, A.; Nag, O.K.; Field, L.D.; Breger, J.C.; Delehanty, J.B. Multifunctional nanoparticle composites: Progress in the use of soft and hard nanoparticles for drug delivery and imaging. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Delehanty, J.B.; Boeneman, K.; Bradburne, C.E.; Robertson, K.; Medintz, I.L. Quantum dots: A powerful tool for understanding the intricacies of nanoparticle-mediated drug delivery. Expert. Opin. Drug Deliv. 2009, 6, 1091–1112. [Google Scholar] [CrossRef] [PubMed]
- Danz, E.D.B.; Skramsted, J.; Henry, N.; Bennett, J.A.; Keller, R.S. Resveratrol prevents doxorubicin cardiotoxicity through mitochondrial stabilization and the Sirt1 pathway. Free Radical. Bio. Med. 2009, 46, 1589–1597. [Google Scholar] [CrossRef] [PubMed]
- Ling, X.; Zhang, S.; Shao, P.; Wang, P.; Ma, X.; Bai, M. Synthesis of a reactive oxygen species responsive heterobifunctional thioketal linker. Tetrahedron Lett. 2015, 56, 5242–5244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shim, M.S.; Xia, Y. A reactive oxygen species (ROS)-responsive polymer for safe, efficient, and targeted gene delivery in cancer cells. Angew. Chem. Int. Ed. 2013, 52, 6926–6929. [Google Scholar] [CrossRef] [PubMed]
- Sagita, E.; Djajadisastra, J.; Mutalib, A. Cytotoxic enhancement of doxorubicin in conjugation with PAMAM G4.5 dendrimer containing gold nanoparticles. Int. J. PharmTech. Res. 2016, 9, 348–356. [Google Scholar]
- Chhikara, B.S.; Jean, N.S.; Mandal, D.; Kumar, A.; Parang, K. Fatty acyl amide derivatives of doxorubicin: Synthesis and in vitro anticancer activities. Eur. J. Med. Chem. 2011, 46, 2037–2042. [Google Scholar]
- Carvalho do Lago, L.C.; Matias, A.C.; Nomura, C.S.; Cerchiaro, G.J. Radical production by hydrogen peroxide/bicarbonate and copper uptake in mammalian cells: Modulation by Cu(II) complexes. Inorg. Biochem. 2011, 105, 189–194. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delehanty, J.B.; Das, S.; Goldberg, E.; Sangtani, A.; Knight, D.A. Synthesis of a Reactive Oxygen Species-Responsive Doxorubicin Derivative. Molecules 2018, 23, 1809. https://doi.org/10.3390/molecules23071809
Delehanty JB, Das S, Goldberg E, Sangtani A, Knight DA. Synthesis of a Reactive Oxygen Species-Responsive Doxorubicin Derivative. Molecules. 2018; 23(7):1809. https://doi.org/10.3390/molecules23071809
Chicago/Turabian StyleDelehanty, James B., Shivani Das, Efram Goldberg, Ajmeeta Sangtani, and D. Andrew Knight. 2018. "Synthesis of a Reactive Oxygen Species-Responsive Doxorubicin Derivative" Molecules 23, no. 7: 1809. https://doi.org/10.3390/molecules23071809
APA StyleDelehanty, J. B., Das, S., Goldberg, E., Sangtani, A., & Knight, D. A. (2018). Synthesis of a Reactive Oxygen Species-Responsive Doxorubicin Derivative. Molecules, 23(7), 1809. https://doi.org/10.3390/molecules23071809