Comprehensive Snake Venomics of the Okinawa Habu Pit Viper, Protobothrops flavoviridis, by Complementary Mass Spectrometry-Guided Approaches
Abstract
:1. Introduction
2. Results
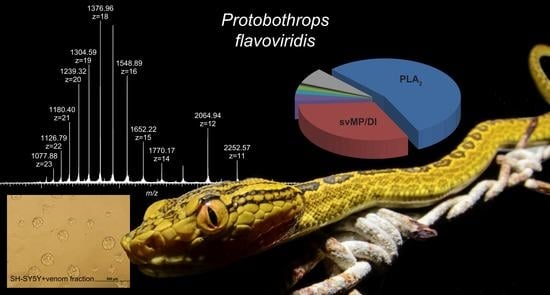
2.1. Top-Down Analysis
2.2. Bottom-Up Analysis
2.3. Bradykinin-Potentiating Peptides and Snake Venom Metalloproteinase Inhibitors
2.4. Cytotoxicity Test
3. Discussion
4. Materials and Methods
4.1. Sample Preparation and System Setup
4.2. Intact Mass Profiling (IMP)
4.3. Top-Down (TD) Venomics
4.4. Bottom-Up (BU) Venomics
4.5. Relative Toxin Quantification
4.6. Data Accessibility
4.7. Cell Culture and In Vitro Cytotoxicity Assay
4.8. Morphological Studies
4.9. Half Maximal Inhibition of Growth (IC50) Determination
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Retief, F.P.; Cilliers, L. Snake and staff symbolism, and healing. Suid-Afrikaanse Tydskrif vir Geneeskunde 2002, 92, 553–556. [Google Scholar] [CrossRef] [PubMed]
- Wake, C.S. The Origin of Serpent-Worship. J. Anthropol. Inst. G. B. Irel. 1873, 2, 373–390. [Google Scholar] [CrossRef]
- Antoniou, S.A.; Antoniou, G.A.; Learney, R.; Granderath, F.A.; Antoniou, A.I. The rod and the serpent: History’s ultimate healing symbol. World J. Surg. 2011, 35, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Stanley, J.W. Snakes: Objects of Religion, Fear and Myth. J. Integr. Biol. 2008, 2, 61–76. [Google Scholar]
- Chippaux, J.-P. Snakebite envenomation turns again into a neglected tropical disease! J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, K.; Mori, A. Literature Survey on Predators of Snakes in Japan. Curr. Herpetol. 2000, 19, 97–111. [Google Scholar] [CrossRef]
- Yasunaga, H.; Horiguchi, H.; Kuwabara, K.; Hashimoto, H.; Matsuda, S. Short report: Venomous snake bites in Japan. Am. J. Trop. Med. Hyg. 2011, 84, 135–136. [Google Scholar] [CrossRef] [PubMed]
- Morokuma, K.; Kobori, N.; Fukuda, T.; Uchida, T.; Sakai, A.; Toriba, M.; Ohkuma, K.; Nakai, K.; Kurata, T.; Takahashi, M. Experimental manufacture of equine antivenom against yamakagashi (Rhabdophis tigrinus). Jpn. J. Infect. Dis. 2011, 64, 397–402. [Google Scholar] [PubMed]
- Tandavanitj, N.; Ota, H.; Cheng, Y.-C.; Toda, M. Geographic genetic structure in two laticaudine sea kraits, Laticauda laticaudata and Laticauda semifasciata (Serpentes Elapidae), in the Ryukyu-Taiwan region as inferred from mitochondrial cytochrome b sequences. Zool. Sci. 2013, 30, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Somaweera, R.; Somaweera, N. Serpents in jars: The snake wine industry in Vietnam. J. Threat. Taxa 2010, 2, 1251–1260. [Google Scholar] [CrossRef]
- Schneider-Poetsch, T.; Takahashi, S.; Jang, J.-H.; Ahn, J.S.; Osada, H. Eighth Korea-Japan Chemical Biology symposium: Chemical biology notes from a small island. J. Antibiot. 2016, 69, 885–888. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.M.; Chun, B.J. Severe Coagulopathy after Ingestion of “Snake Wine”. J. Emerg. Med. 2016, 50, 848–851. [Google Scholar] [CrossRef] [PubMed]
- Tu, M.C.; Wang, H.Y.; Tsai, M.P.; Toda, M.; Lee, W.J.; Zhang, F.J.; Ota, H. Phylogeny, taxonomy, and biogeography of the oriental pitvipers of the genus trimeresurus (reptilia: Viperidae: Crotalinae): A molecular perspective. Zool. Sci. 2000, 17, 1147–1157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chijiwa, T.; Hamai, S.; Tsubouchi, S.; Ogawa, T.; Deshimaru, M.; Oda-Ueda, N.; Hattori, S.; Kihara, H.; Tsunasawa, S.; Ohno, M. Interisland mutation of a novel phospholipase A2 from Trimeresurus flavoviridis venom and evolution of Crotalinae group II phospholipases A2. J. Mol. Evol. 2003, 57, 546–554. [Google Scholar] [CrossRef] [PubMed]
- Shibata, H.; Chijiwa, T.; Hattori, S.; Terada, K.; Ohno, M.; Fukumaki, Y. The taxonomic position and the unexpected divergence of the Habu viper, Protobothrops among Japanese subtropical islands. Mol. Phylogenet. Evol. 2016, 101, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, H.; Enokida, H.; Kawahira, S.; Kagara, I.; Hayami, H.; Nakagawa, M. Acute Kidney Injury and Rhabdomyolysis After Protobothrops flavoviridis Bite: A Retrospective Survey of 86 Patients in a Tertiary Care Center. Am. J. Trop. Med. Hyg. 2016, 94, 474–479. [Google Scholar] [CrossRef] [PubMed]
- Hifumi, T.; Sakai, A.; Kondo, Y.; Yamamoto, A.; Morine, N.; Ato, M.; Shibayama, K.; Umezawa, K.; Kiriu, N.; Kato, H.; et al. Venomous snake bites: Clinical diagnosis and treatment. J. Intensiv. Care 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Fry, B.G. Venomous Reptiles and Their Toxins: Evolution, Pathophysiology, and Biodiscovery; Oxford University Press: New York, NY, USA, 2015. [Google Scholar]
- Bochner, R. Paths to the discovery of antivenom serotherapy in France. J. Venom. Anim. Toxins Incl. Trop. Dis. 2016, 22. [Google Scholar] [CrossRef] [PubMed]
- Lomonte, B.; Calvete, J.J. Strategies in ‘snake venomics’ aiming at an integrative view of compositional, functional, and immunological characteristics of venoms. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 26. [Google Scholar] [CrossRef] [PubMed]
- Mebs, D. Gifttiere: Ein Handbuch für Biologen, Toxikologen, Ärzte und Apotheker, 3rd ed.; Wissenschaftliche Verlagsgesellschaft: Stuttgart, Germany, 2010. [Google Scholar]
- Aird, S.D. Taxonomic distribution and quantitative analysis of free purine and pyrimidine nucleosides in snake venoms. Biochem. Mol. Biol. 2005, 140, 109–126. [Google Scholar] [CrossRef] [PubMed]
- Devi, A. The Protein and Nonprotein Constituents of Snake Venoms. In Venomous Animals and Their Venoms: Venomous Vertebrates, 1st ed.; Bücherl, W., Buckley, E.E., Deulofeu, V., Eds.; Academic Press: New York, NY, USA, 1968; pp. 119–165. [Google Scholar]
- Pimenta, D.C.; Prezoto, B.C.; Konno, K.; Melo, R.L.; Furtado, M.F.; Camargo, A.C.M.; Serrano, S.M.T. Mass spectrometric analysis of the individual variability of Bothrops jararaca venom peptide fraction. Evidence for sex-based variation among the bradykinin-potentiating peptides. Rap. Commun. Mass Spectr. RCM 2007, 21, 1034–1042. [Google Scholar] [CrossRef] [PubMed]
- Mackessy, S.P. Handbook of Venoms and Toxins of Reptiles; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Tasoulis, T.; Isbister, G.K. A Review and Database of Snake Venom Proteomes. Toxins 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.W.; Serrano, S.M.T. Exploring snake venom proteomes: Multifaceted analyses for complex toxin mixtures. Proteomics 2008, 8, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, K.; Ogawa, T.; Oda, N.; Hattori, M.; Sakaki, Y.; Kihara, H.; Ohno, M. Accelerated evolution of Trimeresurus flavoviridis venom gland phospholipase A2 isozymes. Proc. Natl. Acad. Sci. USA 1993, 90, 5964–5968. [Google Scholar] [CrossRef] [PubMed]
- Aoki, N.; Sakiyama, A.; Deshimaru, M.; Terada, S. Identification of novel serum proteins in a Japanese viper: Homologs of mammalian PSP94. Biochem. Biophys. Res. Commun. 2007, 359, 330–334. [Google Scholar] [CrossRef] [PubMed]
- Arlinghaus, F.T.; Eble, J.A. The collagen-binding integrin α2β1 is a novel interaction partner of the Trimeresurus flavoviridis venom protein flavocetin-A. J. Biol. Chem. 2013, 288, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Aird, S.D.; Aggarwal, S.; Villar-Briones, A.; Tin, M.M.-Y.; Terada, K.; Mikheyev, A.S. Snake venoms are integrated systems, but abundant venom proteins evolve more rapidly. BMC Genom. 2015, 16, 647. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, T.; Chijiwa, T.; Oda-Ueda, N.; Ohno, M. Molecular diversity and accelerated evolution of C-type lectin-like proteins from snake venom. Toxicon 2005, 45, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Arlinghaus, F.T.; Eble, J.A. C-type lectin-like proteins from snake venoms. Toxicon 2012, 60, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Atoda, H.; Hyuga, M.; Morita, T. The primary structure of coagulation factor IX/factor X-binding protein isolated from the venom of Trimeresurus flavoviridis. Homology with asialoglycoprotein receptors, proteoglycan core protein, tetranectin, and lymphocyte Fc epsilon receptor for immunoglobulin E. J. Biol. Chem. 1991, 266, 14903–14911. [Google Scholar] [PubMed]
- Taniuchi, Y.; Kawasaki, T.; Fujimura, Y.; Suzuki, M.; Titani, K.; Sakai, Y.; Kaku, S.; Hisamichi, N.; Satoh, N.; Takenaka, T. Flavocetin-A and -B, two high molecular mass glycoprotein Ib binding proteins with high affinity purified from Trimeresurus flavoviridis venom, inhibit platelet aggregation at high shear stress. Biochim. Biophys. Acta 1995, 1244, 331–338. [Google Scholar] [CrossRef]
- Nuyttens, B.P.; Thijs, T.; Deckmyn, H.; Broos, K. Platelet adhesion to collagen. Thromb. Res. 2011, 127, 269. [Google Scholar] [CrossRef]
- Aird, S.D.; Watanabe, Y.; Villar-Briones, A.; Roy, M.C.; Terada, K.; Mikheyev, A.S. Quantitative high-throughput profiling of snake venom gland transcriptomes and proteomes (Ovophis okinavensis and Protobothrops flavoviridis). BMC Genom. 2013, 14, 790. [Google Scholar] [CrossRef] [PubMed]
- Georgieva, D.; Arni, R.K.; Betzel, C. Proteome analysis of snake venom toxins: Pharmacological insights. Exp. Rev. Proteom. 2008, 5, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J.; Juárez, P.; Sanz, L. Snake venomics. Strategy and applications. J. Mass Spectr. 2007, 42, 1405–1414. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J.; Petras, D.; Calderón-Celis, F.; Lomonte, B.; Encinar, J.R.; Sanz-Medel, A. Protein-species quantitative venomics: Looking through a crystal ball. J. Venom. Anim. Toxins Incl. Trop. Dis. 2017, 23, 27. [Google Scholar] [CrossRef] [PubMed]
- Göçmen, B.; Heiss, P.; Petras, D.; Nalbantsoy, A.; Süssmuth, R.D. Mass spectrometry guided venom profiling and bioactivity screening of the Anatolian Meadow Viper, Vipera anatolica. Toxicon 2015, 107, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Hempel, B.-F.; Damm, M.; Göçmen, B.; Karis, M.; Oguz, M.A.; Nalbantsoy, A.; Süssmuth, R.D. Comparative Venomics of the Vipera ammodytes transcaucasiana and Vipera ammodytes montandoni from Turkey Provides Insights into Kinship. Toxins 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.W.; Serrano, S.M.T. Insights into and speculations about snake venom metalloproteinase (SVMP) synthesis, folding and disulfide bond formation and their contribution to venom complexity. FEBS J. 2008, 275, 3016–3030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.Y.; Yoshizumi, K.; Oda, N.; Ohno, M.; Tokunaga, F.; Iwanaga, S.; Kihara, H. Purification and amino acid sequence of basic protein II, a lysine-49-phospholipase A2 with low activity, from Trimeresurus flavoviridis venom. J. Biochem. 1990, 107, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Takeya, H.; Nishida, S.; Nishino, N.; Makinose, Y.; Omori-Satoh, T.; Nikai, T.; Sugihara, H.; Iwanaga, S. Primary structures of platelet aggregation inhibitors (disintegrins) autoproteolytically released from snake venom hemorrhagic metalloproteinases and new fluorogenic peptide substrates for these enzymes. J. Biochem. 1993, 113, 473–483. [Google Scholar] [CrossRef] [PubMed]
- Fox, J.W.; Serrano, S.M.T. Timeline of key events in snake venom metalloproteinase research. J. Proteom. 2009, 72, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Greenbaum, D.; Colangelo, C.; Williams, K.; Gerstein, M. Comparing protein abundance and mRNA expression levels on a genomic scale. Genom. Biol. 2003, 4, 117. [Google Scholar] [CrossRef] [PubMed]
- Margres, M.J.; McGivern, J.J.; Wray, K.P.; Seavy, M.; Calvin, K.; Rokyta, D.R. Linking the transcriptome and proteome to characterize the venom of the eastern diamondback rattlesnake (Crotalus adamanteus). J. Proteom. 2014, 96, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Aird, S.D.; da Silva, N.J.; Qiu, L.; Villar-Briones, A.; Saddi, V.A.; Pires de Campos Telles, M.; Grau, M.L.; Mikheyev, A.S. Coralsnake Venomics: Analyses of Venom Gland Transcriptomes and Proteomes of Six Brazilian Taxa. Toxins 2017, 9. [Google Scholar] [CrossRef] [PubMed]
- Villalta, M.; Pla, D.; Yang, S.L.; Sanz, L.; Segura, A.; Vargas, M.; Chen, P.Y.; Herrera, M.; Estrada, R.; Cheng, Y.F.; et al. Snake venomics and antivenomics of Protobothrops mucrosquamatus and Viridovipera stejnegeri from Taiwan: Keys to understand the variable immune response in horses. J. Proteom. 2012, 75, 5628–5645. [Google Scholar] [CrossRef] [PubMed]
- Gou, P.; Malhotra, A.; Li, P.P.; Pook, C.E.; Creer, S. New evidence on the phylogenetic position of the poorlyknown Asian pitviper Protobothrops kaulbacki (Serpentes: Viperidae: Crotalinae) with a redescription of the species and a revision of the genus Protobothrops. Herpetol. J. 2007, 17, 237–246. [Google Scholar]
- Ota, H.; Toda, M.; Masunaga, G.; Kikukawa, A.; Toda, M. Feral Populations of Amphibians and Reptiles in the Ryukyu Archipelago, Japan. Glob. Envirom. Res. 2004, 8, 133–143. [Google Scholar]
- Hung, D.-Z. Taiwan’s venomous snakebite: Epidemiological, evolution and geographic differences. Trans. R. Soc. Trop. Med. Hyg. 2004, 98, 96–101. [Google Scholar] [CrossRef]
- Liu, C.-C.; Lin, C.-C.; Hsiao, Y.-C.; Wang, P.-J.; Yu, J.-S. Proteomic characterization of six Taiwanese snake venoms: Identification of species-specific proteins and development of a SISCAPA-MRM assay for cobra venom factors. J. Proteom. 2018. [Google Scholar] [CrossRef] [PubMed]
- Rocha e Silva, M.; Beraldo, W.T.; Rosenfeld, G. Bradykinin, a hypotensive and smooth muscle stimulating factor released from plasma globulin by snake venoms and by trypsin. Am. J. Physiol. 1949, 156, 261–273. [Google Scholar] [PubMed]
- Camargo, A.C.M.; Ianzer, D.; Guerreiro, J.R.; Serrano, S.M.T. Bradykinin-potentiating peptides: Beyond captopril. Toxicon 2012, 59, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.H. A Bradykinin-potentiating factor (BPF) present in the venom of Bothrops jaracara. Br. J. Pharm. Chemoth. 1965, 24, 163–169. [Google Scholar] [CrossRef]
- Cushman, D.W.; Cheung, H.S.; Sabo, E.F.; Ondetti, M.A. Design of potent competitive inhibitors of angiotensin-converting enzyme. Carboxyalkanoyl and mercaptoalkanoyl amino acids. Biochemistry 1977, 16, 5484–5491. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, S.; Murayama, N.; Saguchi, K.; Ohi, H.; Fujita, Y.; Camargo, A.C.; Ogawa, T.; Deshimaru, M.; Ohno, M. Bradykinin-potentiating peptides and C-type natriuretic peptides from snake venom. Immunopharmacology 1999, 44, 129–135. [Google Scholar] [CrossRef]
- Wagstaff, S.C.; Favreau, P.; Cheneval, O.; Laing, G.D.; Wilkinson, M.C.; Miller, R.L.; Stöcklin, R.; Harrison, R.A. Molecular characterisation of endogenous snake venom metalloproteinase inhibitors. Biochem. Biophys. Res. Commun. 2008, 365, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.F.; Hung, C.C.; Wu, S.H.; Chiou, S.H. Characterization of three endogenous peptide inhibitors for multiple metalloproteinases with fibrinogenolytic activity from the venom of Taiwan habu (Trimeresurus mucrosquamatus). Biochem. Biophys. Res. Commun. 1998, 248, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.-K.; Yoshii, Y.; Hyodo, A.; Tsurushima, H.; Saito, A.; Harakuni, T.; Li, Y.-P.; Kariya, K.; Nozaki, M.; Morine, N. Apoptotic effect in the glioma cells induced by specific protein extracted from Okinawa Habu (Trimeresurus flavoviridis) venom in relation to oxidative stress. Toxicol. In Vitro 2003, 17, 169–177. [Google Scholar] [CrossRef]
- Murakami, T.; Kamikado, N.; Fujimoto, R.; Hamaguchi, K.; Nakamura, H.; Chijiwa, T.; Ohno, M.; Oda-Ueda, N. A doi:Lys 49 phospholipase A 2 from Protobothrops flavoviridis Venom Induces Caspase-Independent Apoptotic Cell Death Accompanied by Rapid Plasma-Membrane Rupture in Human Leukemia Cells. BioSci. Biotechnol. Biochem. 2014, 75, 864–870. [Google Scholar] [CrossRef]
- Oyama, E.; Takahashi, H. Structures and Functions of Snake Venom Metalloproteinases (SVMP) from Protobothrops venom Collected in Japan. Molecules 2017, 22. [Google Scholar] [CrossRef] [PubMed]
- Dardevet, L.; Rani, D.; Aziz, T.A.E.; Bazin, I.; Sabatier, J.-M.; Fadl, M.; Brambilla, E.; de Waard, M. Chlorotoxin: A helpful natural scorpion peptide to diagnose glioma and fight tumor invasion. Toxins 2015, 7, 1079–1101. [Google Scholar] [CrossRef] [PubMed]
- Ma, R.; Mahadevappa, R.; Kwok, H.F. Venom-based peptide therapy: Insights into anti-cancer mechanism. Oncotarget 2017, 8, 100908–100930. [Google Scholar] [CrossRef] [PubMed]
- Pennington, M.W.; Czerwinski, A.; Norton, R.S. Peptide therapeutics from venom: Current status and potential. Bioorg. Med. Chem. 2017. [Google Scholar] [CrossRef] [PubMed]
- Petras, D.; Heiss, P.; Harrison, R.A.; Süssmuth, R.D.; Calvete, J.J. Top-down venomics of the East African green mamba, Dendroaspis angusticeps, and the black mamba, Dendroaspis polylepis, highlight the complexity of their toxin arsenals. J. Proteom. 2016, 146, 148–164. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Muth, T.; Weilnböck, L.; Rapp, E.; Huber, C.G.; Martens, L.; Vaudel, M.; Barsnes, H. DeNovoGUI: An open source graphical user interface for de novo sequencing of tandem mass spectra. J. Proteom. Res. 2014, 13, 1143–1146. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J. Proteomic tools against the neglected pathology of snake bite envenoming. Exp. Rev. Proteom. 2011, 8, 739–758. [Google Scholar] [CrossRef] [PubMed]
- Calvete, J.J. Next-generation snake venomics: Protein-locus resolution through venom proteome decomplexation. Exp. Rev. Proteom. 2014, 11, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Vizcaíno, J.A.; Deutsch, E.W.; Wang, R.; Csordas, A.; Reisinger, F.; Ríos, D.; Dianes, J.A.; Sun, Z.; Farrah, T.; Bandeira, N.; et al. ProteomeXchange provides globally coordinated proteomics data submission and dissemination. Nat. Biotechnol. 2014, 32, 223–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Sample Availability: Samples of the venom compounds are not available from the authors. |





| Toxin Families | Protein ID | Highest E-Value | NCBI Accession No. | No. of Sequence Proteoforms |
|---|---|---|---|---|
| PLA2 | basic phospholipase A2 BP-III | 8.21 × 10−16 | C7G1G6.1 | 20 |
| basic phospholipase A2 PL-B | 7.42 × 10−15 | P59265.1 | 11 | |
| phospholipase A2 | 7.85 × 10−10 | 1202299A | 11 | |
| basic phospholipase A2 PL-X | 8.35 × 10−10 | P06860.1 | 5 | |
| basic phospholipase A2 PLA-A | 2.98 × 10−14 | P59264.1 | 3 | |
| basic phospholipase A2 BP-II | 3.70 × 10−6 | P0DJJ9.1 | 2 | |
| basic phospholipase A2 BP-I | 8.90 × 10−6 | P0DJJ8.1 | 2 | |
| phospholipase A2 | 4.17 × 10−3 | BAA01561.1 | 2 | |
| basic phospholipase A2 PL-Y | 2.37 × 10−8 | Q90Y77.1 | 1 | |
| svMP | zinc metalloproteinase/disintegrin | 1.29 × 10−6 | P18619.2 | 3 |
| P-II metalloprotease | 5.12 × 10−5 | BAN89360.1 | 1 | |
| snake venom metalloproteinase HR2a | 2.16 × 10−3 | P14530.3 | 1 | |
| snake venom metalloproteinase trimerelysin-II | 3.12 × 10−3 | P20165.3 | 1 | |
| DI | disintegrin CTF-II | 4.91 × 10−7 | P23323.1 | 3 |
| cytotoxic factor | 9.96 × 10−8 | AAB19943.1 | 1 | |
| disintegrin triflavin | 4.67 × 10−4 | P21859.1 | 1 | |
| LAAO | l-amino acid oxidase | 1.21 × 10−6 | BAP39950.1 | 1 |
| l-amino acid oxidase | 1.28 × 10−4 | BAN82013.1 | 1 | |
| CTL | coagulation factor IX/X-binding protein | 1.21 × 10−6 | 1IXX_F | 1 |
| BPP related | Bradykinin-potentiating and C-type natriuretic peptides | 4.93 × 10−6 | BAP39952.1 | 1 |
| CRISP | CRISP Family Ca-Channel Blocker | 7.52 × 10−5 | 1WVR_A | 1 |
| Fraction | Toxin Family | Isoform Identity by | UniProt-ID for IMP | Average Mass in Da | ||
|---|---|---|---|---|---|---|
| BU | IMP | Observed | Theoretical | |||
| 16 | PLA2 | BPII | BPII | P0DJJ9 | 13,752.4 | 13,752.1 |
| BPI | P0DJJ8 | 13,753.1 | ||||
| 17 | PLA2 | PLA * | PLA-N(O) | S6BAM8 | 14,020.2 | 14,019.2 |
| 18 | PLA2 | PLA * | PL-Y | Q90Y77 | 13,944.3 | 13,945.2 |
| 20 | CRISP | triflin | triflin-II | T2HP25 | 24,767.2 | 24,767.9 |
| 22 | PLA2 | PLA2 1 | PLA2 1 | P06859 | 13,764.0 | 13,764.6 |
| 25 | svSP | flavoxobin | flavoxobin | P05620 | 25,686.4 | 25,687.4 |
| Cell Line | P. flavoviridis IC50 Values in µg/mL | Doxorubicin IC50 Values in µg/mL |
|---|---|---|
| HEK-293 | 1.02 ± 0.02 | 0.002 ± 0.001 |
| SH-SY5Y | 4.68 ± 0.92 | 0.06 ± 0.02 |
| MDA-MB-231 | 22.84 ± 2.51 | 4.98 ± 0.28 |
| A549 | 38.51 ± 0.13 | 1.03 ± 0.37 |
| PANC1 | >50 | 0.05 ± 0.01 |
| HeLa | 24.78 ± 0.77 | 1.03 ± 0.25 |
| PC-3 | 51.55 ± 2.79 | 2.02 ± 0.46 |
| HPLC Fraction | Molecular Mass in Da | IC50 | |
|---|---|---|---|
| in µg/mL | in µM | ||
| 4 | 443 | 0.680 ± 0.002 | 1.53 ± 0.01 |
| 5 | 1233 | 4.60 ± 0.24 | 3.73 ± 0.19 |
| 6 | 429 | 9.19 ± 1.22 | 21.42 ± 2.84 |
| 7/8 | 443 | 0.87 ± 0.05 | 1.96 ± 0.11 |
| 10 | 7508 | 49.63 ± 2.46 | 6.61 ± 0.33 |
| 11 | 7956 | 45.46 ± 7.45 | 5.71 ± 0.94 |
| 13 | 7956 | 17.94 ± 2.96 | 2.25 ± 0.37 |
| 14 | 7621 | 4.02 ± 0.24 | 0.53 ± 0.03 |
| 15 | 14,000 * | 2.18 ± 0.12 | 0.16 ± 0.01 |
| 16 | 13,752 | 30.57 ± 1.50 | 2.22 ± 0.11 |
| 17 | 14,020 | 23.96 ± 2.55 | 1.71 ± 0.18 |
| 18 | 13,944 | 38.82 ± 2.82 | 2.78 ± 0.20 |
| 19 | 14,000 * | 17.25 ± 0.98 | 1.23 ± 0.07 |
| 20 | 24,767 | 15.57 ± 1.10 | 0.63 ± 0.04 |
| Doxorubicin | 544 | 0.83 ± 0.20 | 1.53 ± 0.37 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Damm, M.; Hempel, B.-F.; Nalbantsoy, A.; Süssmuth, R.D. Comprehensive Snake Venomics of the Okinawa Habu Pit Viper, Protobothrops flavoviridis, by Complementary Mass Spectrometry-Guided Approaches. Molecules 2018, 23, 1893. https://doi.org/10.3390/molecules23081893
Damm M, Hempel B-F, Nalbantsoy A, Süssmuth RD. Comprehensive Snake Venomics of the Okinawa Habu Pit Viper, Protobothrops flavoviridis, by Complementary Mass Spectrometry-Guided Approaches. Molecules. 2018; 23(8):1893. https://doi.org/10.3390/molecules23081893
Chicago/Turabian StyleDamm, Maik, Benjamin-Florian Hempel, Ayse Nalbantsoy, and Roderich D. Süssmuth. 2018. "Comprehensive Snake Venomics of the Okinawa Habu Pit Viper, Protobothrops flavoviridis, by Complementary Mass Spectrometry-Guided Approaches" Molecules 23, no. 8: 1893. https://doi.org/10.3390/molecules23081893
APA StyleDamm, M., Hempel, B. -F., Nalbantsoy, A., & Süssmuth, R. D. (2018). Comprehensive Snake Venomics of the Okinawa Habu Pit Viper, Protobothrops flavoviridis, by Complementary Mass Spectrometry-Guided Approaches. Molecules, 23(8), 1893. https://doi.org/10.3390/molecules23081893