Lignan Glycosides and Flavonoid Glycosides from the Aerial Portion of Lespedeza cuneata and Their Biological Evaluations
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation of the Compounds
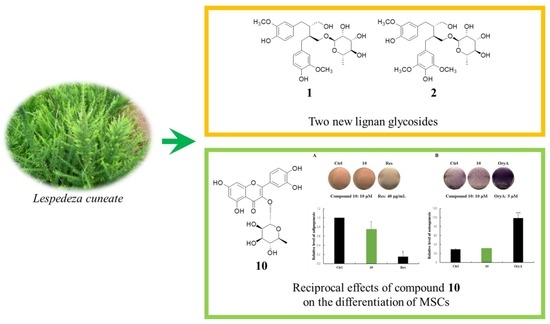
2.2. Structure Elucidation of the Compounds
2.3. Cytotoxic Activity of Isolated Compounds against Human Tumor Cell Lines
2.4. Antiviral Activity of the Isolated Compounds against PR8, HRV1B, and CVB3 Infection
2.5. Regulatory Effects of Compound 10 on Differentiation into Adipocytes and Osteoblasts
3. Materials and Methods
3.1. Plant Material
3.2. Extraction and Isolation
3.2.1. (+)-Secoisolariciresinol-O-α-l-rhamnopyranoside (1)
3.2.2. (+)-Seco-5’-methoxy-isolariciresinol-9’-O-α-l-rhamnopyranoside (2)
3.3. Enzymatic Hydrolysis of Compounds 1,2
3.4. Cytotoxicity Assay
3.5. Antiviral Activity Assay
3.6. Oil Red OStaining
3.7. Alkaline Phosphatase (ALP) Staining and Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kim, M.S.; Sharma, B.R.; Rhyu, D.Y. Beneficial effect of Lespedeza cuneata (G. Don) water extract on streptozotocin-induced type 1 diabetes and cytokine-induced beta-cell damage. Nat. Prod. Sci. 2016, 22, 175–179. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, J.; Yang, J.; Li, C.; Ma, J.; Zhang, D.; Zhang, D. Two new phenylpropanoid glycosides from the aerial parts of Lespedeza cuneata. Acta Pharm. Sin. B 2016, 6, 564–567. [Google Scholar] [CrossRef] [PubMed]
- Min, J.Y.; Shim, S.H. Chemical constituents from Lespedeza cuneata G. Don (Leguminosae). Biochem. Syst. Ecol. 2016, 66, 293–296. [Google Scholar] [CrossRef]
- Lee, H.; Jung, J.Y.; Hwangbo, M.; Ku, S.K.; Kim, Y.W.; Jee, S.Y. Anti-inflammatory effects of Lespedeza cuneata in vivo and in vitro. Korea J. Herbol. 2013, 28, 83–92. [Google Scholar] [CrossRef]
- Zhang, C.F.; Zhou, J.; Yang, J.Z.; Li, C.J.; Ma, J.; Zhang, D.; Li, L.; Zhang, D.M. Three new lignanosides from the aerial parts of Lespedeza cuneata. J. Asian Nat. Prod. Res. 2016, 18, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Kang, K.; Jho, E.H.; Jung, Y.J.; Nho, C.W.; Um, B.H.; Pan, C.H. Hepatoprotective effect of flavonoid glycosides from Lespedeza cuneata against oxidative stress induced by tert-butyl hyperoxide. Phytother. Res. 2011, 25, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Park, H.M.; Hong, J.H. Physiological activities of Lespedeza cuneata extracts. Korea J. Food Preserv. 2014, 21, 844–850. [Google Scholar] [CrossRef]
- Deng, F.; Chang, J.; Zhang, J.S. New flavonoids and other constituents from Lespedeza cuneata. J. Asian Nat. Prod. Res. 2007, 9, 655–658. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, C.J.; Yang, J.Z.; Ma, J.; Wu, L.Q.; Wang, W.J.; Zhang, D.M. Phenylpropanoid and lignan glycosides from the aerial parts of Lespedeza cuneata. Phytochemistry 2016, 121, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Ye, J.; Xie, Y.G.; Pan, Y.P.; Zheng, Y.; Qian, X.P.; Jin, H.Z. A new phenyldilactone from Lespedeza cuneata. J. Asian Nat. Prod. Res. 2016, 18, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Yoo, G.; Park, S.J.; Lee, T.H.; Yang, H.; Baek, Y.S.; Kim, N.; Kim, Y.J.; Kim, S.H. Flavonoids isolated from Lespedeza cuneata G. Don and their inhibitory effects on nitric oxide production in lipopolysaccharide-stimulated BV-2 microglia cells. Pharmacogn. Mag. 2015, 11, 651–656. [Google Scholar] [PubMed]
- Yu, J.S.; Baek, J.; Park, H.B.; Moon, E.; Kim, S.Y.; Choi, S.U.; Kim, K.H. A new rearranged eudesmane sesquiterpene and bioactive sesquiterpenes from the twigs of Lindera glauca (Sieb. et Zucc.) Blume. Arch. Pharm. Res. 2016, 39, 1628–1634. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Kang, K.S.; Yu, J.S.; Woo, J.Y.; Hwang, G.S.; Eom, D.W.; Baek, S.H.; Lee, H.L.; Kim, K.H.; Yamabe, N. Protective effect of Korean Red Ginseng against FK506-induced damage in LLC-PK1 cells. J. Ginseng Res. 2017, 41, 284–289. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; So, H.M.; Roh, H.S.; Kim, J.; Yu, J.S.; Lee, S.; Seok, S.; Pang, C.; Baek, K.H.; Kim, K.H. Vulpinic acid contributes to the cytotoxicity of Pulveroboletus ravenelii to human cancer cells by inducing apoptosis. RSC Adv. 2017, 7, 35297–35304. [Google Scholar] [CrossRef]
- Baek, J.; Lee, D.; Lee, T.K.; Song, J.H.; Lee, J.S.; Lee, S.; Yoo, S.W.; Kang, K.S.; Moon, E.; Lee, S.; et al. (−)-9’-O-(α-l-Rhamnopyranosyl)lyoniresinol from Lespedeza cuneata suppresses ovarian cancer cell proliferation through induction of apoptosis. Bioorg. Med. Chem. Lett. 2018, 28, 122–128. [Google Scholar] [CrossRef] [PubMed]
- Sugahara, T.; Yamauchi, S.; Kondo, A.; Ohno, F.; Tominaga, S.; Nakashima, Y.; Kishida, T.; Akiyama, K.; Maruyama, M. First stereoselective synthesis of meso-secoisolariciresinol and comparison of its biological activity with (+) and (−)-secoisolariciresinol. Biosci. Biotechnol. Biochem. 2007, 71, 2962–2968. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Koike, K.; Liu, L.; Lin, L.; Fu, X.; Chen, Y.; Nikaido, T. New lignan glucosides from the stems of Tinospora sinensis. Chem. Pharm. Bull. 2004, 52, 638–640. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.R.; Lee, D.; Benndorf, R.; Jung, W.H.; Beemelmanns, C.; Kang, K.S.; Kim, K.H. Termisoflavones A-C., isoflavonoid glycosides from termite-associated Streptomyces sp. RB1. J. Nat. Prod. 2016, 79, 3072–3078. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhu, Q. Pregnane glycoside, lignan glycosides, triterpene glycosyl ester and flavonoid glycosides from Rubus amabilis. Planta Med. 2001, 67, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Subedi, L.; Kim, S.Y.; Choi, S.U.; Kim, K.H.; Lee, K.R. Lignan glycosides from the twigs of Chaenomeles sinensis and their biological activities. J. Nat. Prod. 2015, 78, 1174–1178. [Google Scholar] [CrossRef] [PubMed]
- He, W.J.; Fu, Z.H.; Zeng, G.Z.; Zhang, Y.M.; Han, H.J.; Yan, H.; Ji, C.J.; Chu, H.B.; Tan, N.H. Terpene and lignan glycosides from the twigs and leaves of an endangered conifer, Cathaya argyrophylla. Phytochemistry 2012, 83, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Kil, Y.S.; Kim, S.M.; Kang, U.; Chung, H.Y.; Seo, E.K. Peroxynitrite-scavenging glycosides from the stem bark of Catalpa ovata. J. Nat. Prod. 2017, 80, 2240–2251. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Nakashima, T.; Ueda, T.; Tomii, K.; Kouno, I. Facile discrimination of aldose enantiomers by reversed-phase HPLC. Chem. Pharm. Bull. 2007, 55, 899–901. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.K.; Suh, W.S.; Kim, K.H.; Kim, S.Y.; Lee, K.R. Phytochemical constituents of Salsola komarovii and their effects on NGF induction. Nat. Prod. Sci. 2014, 20, 95–101. [Google Scholar]
- Rayyan, S.; Fossen, T.; Nateland, H.S.; Andersen, O.M. Isolation and identification of flavonoids, including flavone rotamers, from the herbal drug ‘Crataegi folium cum flore’ (hawthorn). Phytochem. Anal. 2005, 16, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K. Antioxidative constituents from the twigs of Vitex rotundifolia. Biomol. Ther. 2009, 17, 412–417. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, J.S.; Lee, S.Y.; Bae, K.H.; Kang, S.S. Chemical constituents of Lathyrus davidii. Nat. Prod. Sci. 2008, 14, 281–288. [Google Scholar]
- Han, J.T.; Bang, M.H.; Chun, O.K.; Kim, D.O.; Lee, C.Y.; Baek, N.I. Flavonol glycosides from the aerial parts of Aceriphyllum rossii and their antioxidant activities. Arch. Pharm. Res. 2004, 27, 390–395. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.Y.; Tao, W.W.; Duan, J.A. Antithrombotic flavonoids from the faeces of Trogopterus xanthipes. Nat. Prod. Res. 2010, 24, 1843–1849. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.H.; Son, Y.K.; Han, Y.N. Tissue factor inhibitory flavonoids from the fruits of Chaenomeles sinensis. Arch. Pharm. Res. 2002, 25, 842–850. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.K.; Kim, Y.C.; Takaya, Y.; Terashima, K.; Niwa, M. Novel flavonol glycoside, 7-O-methyl mearnsitrin, from Sageretia theezans and its antioxidant effect. J. Agric. Food Chem. 2004, 52, 4664–4668. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Woo, E.R.; Park, H. A novel lignan and flavonoids from Polygonum aviculare. J. Nat. Prod. 1994, 57, 581–586. [Google Scholar] [CrossRef]
- Torres-Mendoza, D.; González, J.; Ortega-Barría, E.; Heller, M.V.; Capson, T.L.; McPhail, K.; Gerwick, W.H.; Cubilla-Rios, L. Weakly antimalarial flavonol arabinofuranosides from Calycolpus warszewiczianus. J. Nat. Prod. 2006, 69, 826–828. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.J.; Chow, J.M.; Chuang, S.E.; Chang, C.L.; Yan, M.D.; Lee, H.L.; Lai, I.C.; Lin, P.C.; Lai, G.M. Induction of Forkhead Class box O3a and apoptosis by a standardized ginsenoside formulation, KG-135, is potentiated by autophagy blockade in A549 human lung cancer cells. J. Ginseng Res. 2017, 41, 247–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, S.T.; Huang, Y.T.; Hsiung, H.Y.; Huang, W.H.; Yao, C.W.; Lee, A.R. Novel daidzein analogs and their in vitro anti-influenza activities. Chem. Biodivers. 2015, 12, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Argenta, D.F.; Silva, I.T.; Bassani, V.L.; Koester, L.S.; Teixeira, H.F.; Simões, C.M. Antiherpes evaluation of soybean isoflavonoids. Arch. Virol. 2015, 160, 2335–2342. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wu, Z.; Du, J.; Hu, Y.F.; Liu, L.; Yang, F.; Jin, Q. Anti-Japanese-encephalitis-viral effects of kaempferol and daidzin and their RNA-binding characteristics. PLoS ONE 2012, 7, e30259. [Google Scholar] [CrossRef] [PubMed]
- Sui, B.D.; Hu, C.H.; Zheng, C.X.; Jin, Y. Microenvironmental views on mesenchymal stem cell differentiation in aging. J. Den. Res. 2016, 95, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Yeo, S.G.; Hong, E.H.; Lee, B.R.; Kim, J.W.; Kim, J.; Jeong, H.; Kwon, Y.; Kim, H.; Lee, S.; et al. Antiviral activity of hederasaponin B from hedera helix against enterovirus 71 subgenotypes C3 and C4a. Biomol. Ther. 2014, 22, 41–46. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |

 ) and key HMBC (
) and key HMBC (  ) correlations for 1 and 2.
) correlations for 1 and 2.

| Position | 1 | 2 | ||||
|---|---|---|---|---|---|---|
| δH | δC | δH | δC | |||
| 1 | 133.6 | s | 132.2 | s | ||
| 2 | 6.56 d (2.0) | 113.0 | d | 6.54 d (2.0) | 111.9 | d |
| 3 | 6.67 α d (8.0) | 115.5 | d | 6.65 d (8.0) | 114.2 | d |
| 4 | 145.4 | s | 144.5 | s | ||
| 5 | 148.9 | s | 147.5 | s | ||
| 6 | 6.53 dd (8.0, 2.0) | 122.6 | d | 6.52 dd (8.0, 2.0) | 121.3 | d |
| 7 | 2.67 dd (14.0, 7.0); 2.56 dd (14.0, 8.5) | 35.6 | t | 2.69 dd (14.0, 6.5); 2.53 dd (14.0, 9.0) | 34.5 | t |
| 8 | 1.94 m | 44.1 | d | 1.92 m | 42.5 | d |
| 9 | 3.69 m; 3.48 dd (11.0, 7.0) | 62.6 | t | 3.71 m; 3.48 dd (11.0, 7.0) | 61.2 | t |
| 1’ | 133.6 | s | 131.4 | s | ||
| 2’ | 6.54 d (2.0) | 113.0 | d | 6.28 s | 105.3 | d |
| 3’ | 148.8 | s | 147.6 | s | ||
| 4’ | 145.4 | s | 133.4 | s | ||
| 5’ | 6.66 α d (8.0) | 115.5 | d | 147.6 | s | |
| 6’ | 6.53 dd (8.0, 2.0) | 122.6 | d | 6.28 s | 105.3 | d |
| 7’ | 2.60 m | 35.8 | t | 2.60 m | 35.2 | t |
| 8’ | 2.07 m | 40.7 | d | 2.08 m | 39.3 | d |
| 9’ | 3.77 dd (10.0, 6.0); 3.33 m | 69.7 | t | 3.79 dd (10.0, 6.0); 3.35 m | 67.9 | t |
| 1’’ | 4.63 d (1.5) | 102.0 | d | 4.64 d (1.5) | 100.7 | d |
| 2’’ | 3.82 dd (3.5, 1.5) | 72.2 | d | 3.81 dd (3.5, 1.5) | 71.0 | d |
| 3’’ | 3.68 dd (9.5, 3.5) | 72.4 | d | 3.68 dd (9.5, 3.5) | 71.1 | d |
| 4’’ | 3.38 t (9.5) | 73.7 | d | 3.38 t (9.5) | 72.5 | d |
| 5’’ | 3.62 dq (9.5, 6.0) | 69.9 | d | 3.62 dq (9.5, 6.0) | 68.7 | d |
| 6’’ | 1.25 d (6.0) | 17.8 | q | 1.25 d (6.0) | 16.5 | q |
| 3-OCH3 | 3.73 β s | 55.8 | q | 3.72 s | 54.7 | q |
| 3’-OCH3 | 3.74 β s | 55.8 | q | 3.74 s | 55.1 | q |
| 5’-OCH3 | 3.74 s | 55.1 | q | |||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baek, J.; Lee, T.K.; Song, J.-H.; Choi, E.; Ko, H.-J.; Lee, S.; Choi, S.U.; Lee, S.; Yoo, S.-W.; Kim, S.-H.; et al. Lignan Glycosides and Flavonoid Glycosides from the Aerial Portion of Lespedeza cuneata and Their Biological Evaluations. Molecules 2018, 23, 1920. https://doi.org/10.3390/molecules23081920
Baek J, Lee TK, Song J-H, Choi E, Ko H-J, Lee S, Choi SU, Lee S, Yoo S-W, Kim S-H, et al. Lignan Glycosides and Flavonoid Glycosides from the Aerial Portion of Lespedeza cuneata and Their Biological Evaluations. Molecules. 2018; 23(8):1920. https://doi.org/10.3390/molecules23081920
Chicago/Turabian StyleBaek, Jiwon, Tae Kyoung Lee, Jae-Hyoung Song, Eunyong Choi, Hyun-Jeong Ko, Sanghyun Lee, Sang Un Choi, Seong Lee, Sang-Woo Yoo, Seon-Hee Kim, and et al. 2018. "Lignan Glycosides and Flavonoid Glycosides from the Aerial Portion of Lespedeza cuneata and Their Biological Evaluations" Molecules 23, no. 8: 1920. https://doi.org/10.3390/molecules23081920
APA StyleBaek, J., Lee, T. K., Song, J. -H., Choi, E., Ko, H. -J., Lee, S., Choi, S. U., Lee, S., Yoo, S. -W., Kim, S. -H., & Kim, K. H. (2018). Lignan Glycosides and Flavonoid Glycosides from the Aerial Portion of Lespedeza cuneata and Their Biological Evaluations. Molecules, 23(8), 1920. https://doi.org/10.3390/molecules23081920