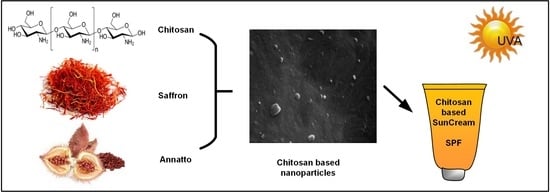
Chitosan Nanoparticles with Encapsulated Natural and UF-Purified Annatto and Saffron for the Preparation of UV Protective Cosmetic Emulsions
Abstract
:1. Introduction
2. Results and Discussion
2.1. CS-Based Nanoparticles
2.2. Sunscreen Emulsions
2.2.1. Emulsion Stability Results
2.2.2. Emulsion SPF
3. Materials and Methods
3.1. Materials
3.2. Extraction and Ultrafiltration of Saffron
3.3. Preparation of CS Nanoparticles
3.4. Characterization of CS Nanoparticles
3.4.1. Morphological Characterization of Nanoparticles
3.4.2. Size Measurements of Nanoparticles
3.4.3. Wide Angle X-ray Diffractometry (WAXD)
3.4.4. Fourier Transformation Infrared Spectroscopy (FTIR)
3.4.5. Thermogravimetric Analysis (TGA)
3.4.6. Evaluation of Additive Encapsulation
3.4.7. In Vitro Cytotoxicity Study
3.5. Preparation of Emulsions
3.6. Characterization of Emulsions
3.6.1. Emulsion Stability
3.6.2. SPF Determination
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cadet, J.; Douki, T.; Ravanat, J.L. Oxidatively generated damage to cellular DNA by UVB and UVA radiation. Photochem. Photobiol. 2015, 91, 140–155. [Google Scholar] [CrossRef] [PubMed]
- Agbai, O.N.; Buster, K.; Sanchez, M.; Hernandez, C.; Kundu, R.V.; Chiu, M.; Roberts, W.E.; Draelos, Z.D.; Bhushan, R.; Taylor, S.C.; et al. Skin cancer and photoprotection in people of color: A review and recommendations for physicians and the public. J. Am. Acad. Dermatol. 2014, 70, 748–762. [Google Scholar] [CrossRef] [PubMed]
- Shetty, P.K.; Venuvanka, V.; Jagani, H.V.; Chethan, G.H.; Ligade, V.S.; Musmade, P.B.; Nayak, U.Y.; Reddy, M.S.; Kalthur, G.; Udupa, N.; et al. Development and evaluation of sunscreen creams containing morin-encapsulated nanoparticles for enhanced UV radiation protection and antioxidant activity. Int. J. Nanomed. 2015, 10, 6477–6491. [Google Scholar]
- Serpone, N.; Dondi, D.; Albini, A. Inorganic and organic UV filters: Their role and efficacy in sunscreens and suncare products. Inorg. Chim. Acta 2007, 360, 794–802. [Google Scholar] [CrossRef]
- Sarkany, R. Sun protection strategies. Medicine 2017, 45, 444–447. [Google Scholar] [CrossRef]
- Maske, P.P.; Lokapure, S.G.; Nimbalkar, D.; Malavi, S.; D’Souza, J.I. In vitro determination of sun protection factor and chemical stability of Rosa kordesii extract gel. J. Pharm. Res. 2013, 7, 520–524. [Google Scholar] [CrossRef]
- Binks, B.P.; Fletcher, P.D.I.; Johnson, A.J.; Marinopoulos, I.; Crowther, J.; Thompson, M.A. How the sun protection factor (SPF) of sunscreen films change during solar irradiation. J. Photochem. Photobiol. A Chem. 2017, 333, 186–199. [Google Scholar] [CrossRef]
- Lin, C.-C.; Lin, W.-J. Sun Protection Factor Analysis of Sunscreens Containing Titanium Dioxide Nanoparticles. J. Food Drug Anal. 2011, 19, 1–8. [Google Scholar]
- Newman, M.D.; Stotland, M.; Ellis, J.I. The safety of nanosized particles in titanium dioxide- and zinc oxide-based sunscreens. J. Am. Acad. Dermatol. 2009, 61, 685–692. [Google Scholar] [CrossRef] [PubMed]
- AbdElhady, M.M. Preparation and Characterization of Chitosan/Zinc Oxide Nanoparticles for Imparting Antimicrobial and UV Protection to Cotton Fabric. Int. J. Carbohydr. Chem. 2012, 2012, 6. [Google Scholar] [CrossRef]
- Chung, K.-H.; Jung, H.-Y.; Lee, Y.-W.; Lee, K.-Y. Preparation of TiO2-loaded nanocapsules and their sun protection behaviors. J. Ind. Eng. Chem. 2010, 16, 261–266. [Google Scholar] [CrossRef]
- Hirano, S.; Noishiki, Y. The blood compatibility of chitosan and N-acylchitosans. J. Biomed. Mater. Res. 1985, 19, 413–417. [Google Scholar] [CrossRef] [PubMed]
- Koukaras, E.N.; Papadimitriou, S.A.; Bikiaris, D.N.; Froudakis, G.E. Insight on the Formation of Chitosan Nanoparticles through Ionotropic Gelation with Tripolyphosphate. Mol. Pharm. 2012, 9, 2856–2862. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, S.; Bikiaris, D.; Avgoustakis, K.; Karavas, E.; Georgarakis, M. Chitosan nanoparticles loaded with dorzolamide and pramipexole. Carbohydr. Polym. 2008, 73, 44–54. [Google Scholar] [CrossRef]
- Archana, D.; Dutta, J.; Dutta, P.K. Evaluation of chitosan nano dressing for wound healing: Characterization, in vitro and in vivo studies. Int. J. Biol. Macromol. 2013, 57, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, R.; Prabaharan, M.; Sudheesh Kumar, P.T.; Nair, S.V.; Tamura, H. Biomaterials based on chitin and chitosan in wound dressing applications. Biotechnol. Adv. 2011, 29, 322–337. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.T.; Lakshmanan, V.K.; Anilkumar, T.V.; Ramya, C.; Reshmi, P.; Unnikrishnan, A.G.; Nair, S.V.; Jayakumar, R. Flexible and microporous chitosan hydrogel/nano ZnO composite bandages for wound dressing: In vitro and in vivo evaluation. ACS Appl. Mater. Interfaces 2012, 4, 2618–2629. [Google Scholar] [CrossRef] [PubMed]
- Madihally, S.V.; Matthew, H.W. Porous chitosan scaffolds for tissue engineering. Biomaterials 1999, 20, 1133–1142. [Google Scholar] [CrossRef]
- Ravi Kumar, M.N.V. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Kean, T.; Thanou, M. Biodegradation, biodistribution and toxicity of chitosan. Adv. Drug Deliv. Rev. 2010, 62, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-C.; Mi, F.-L.; Liao, Z.-X.; Hsiao, C.-W.; Sonaje, K.; Chung, M.-F.; Hsu, L.-W.; Sung, H.-W. Recent advances in chitosan-based nanoparticles for oral delivery of macromolecules. Adv. Drug Deliv. Rev. 2013, 65, 865–879. [Google Scholar] [CrossRef] [PubMed]
- Casettari, L.; Illum, L. Chitosan in nasal delivery systems for therapeutic drugs. J. Control. Release 2014, 190, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Aungst, B.J. Absorption Enhancers: Applications and Advances. AAPS J. 2012, 14, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Koutroumanis, K.P.; Avgoustakis, K.; Bikiaris, D. Synthesis of cross-linked N-(2-carboxybenzyl)chitosan pH sensitive polyelectrolyte and its use for drug controlled delivery. Carbohydr. Polym. 2010, 82, 181–188. [Google Scholar] [CrossRef]
- Nanaki, S.; Tseklima, M.; Christodoulou, E.; Triantafyllidis, K.; Kostoglou, M.; Bikiaris, D. Thiolated Chitosan Masked Polymeric Microspheres with Incorporated Mesocellular Silica Foam (MCF) for Intranasal Delivery of Paliperidone. Polymers 2017, 9, 617. [Google Scholar] [CrossRef]
- Nanaki, S.G.; Koutsidis, I.A.; Koutri, I.; Karavas, E.; Bikiaris, D. Miscibility study of chitosan/2-hydroxyethyl starch blends and evaluation of their effectiveness as drug sustained release hydrogels. Carbohydr. Polym. 2012, 87, 1286–1294. [Google Scholar] [CrossRef]
- Siafaka, P.I.; Titopoulou, A.; Koukaras, E.N.; Kostoglou, M.; Koutris, E.; Karavas, E.; Bikiaris, D.N. Chitosan derivatives as effective nanocarriers for ocular release of timolol drug. Int. J. Pharm. 2015, 495, 249–264. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, K.; Singh, S.K.; Mishra, D.N. Chitosan nanoparticles: A promising system in novel drug delivery. Chem. Pharm. Bull. 2010, 58, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Prabaharan, M. Chitosan-based nanoparticles for tumor-targeted drug delivery. Int. J. Biol. Macromol. 2015, 72, 1313–1322. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.J.; Zeng, Z.W.; Xiao, R.Z.; Xie, T.; Zhou, G.L.; Zhan, X.R.; Wang, S.L. Recent advances of chitosan nanoparticles as drug carriers. Int. J. Nanomed. 2011, 6, 765–774. [Google Scholar]
- Panos, I.; Acosta, N.; Heras, A. New drug delivery systems based on chitosan. Curr. Drug Discov. Technol. 2008, 5, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Koukaras, E.N.; Papadimitriou, S.A.; Bikiaris, D.N.; Froudakis, G.E. Properties and energetics for design and characterization of chitosan nanoparticles used for drug encapsulation. RSC Adv. 2014, 4, 12653–12661. [Google Scholar] [CrossRef]
- Zastrow, L.; Meinke, M.C.; Albrecht, S.; Patzelt, A.; Lademann, J. From UV Protection to Protection in the Whole Spectral Range of the Solar Radiation: New Aspects of Sunscreen Development. In Ultraviolet Light in Human Health, Diseases and Environment; Ahmad, S.I., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 311–318. [Google Scholar]
- Meinke, M.C.; Syring, F.; Schanzer, S.; Haag, S.F.; Graf, R.; Loch, M.; Gersonde, I.; Groth, N.; Pflucker, F.; Lademann, J. Radical protection by differently composed creams in the UV/VIS and IR spectral ranges. Photochem. Photobiol. 2013, 89, 1079–1084. [Google Scholar] [CrossRef] [PubMed]
- Lademann, J.; Schanzer, S.; Jacobi, U.; Schaefer, H.; Pflucker, F.; Driller, H.; Beck, J.; Meinke, M.; Roggan, A.; Sterry, W. Synergy effects between organic and inorganic UV filters in sunscreens. J. Biomed. Opt. 2005, 10, 014008. [Google Scholar] [CrossRef] [PubMed]
- Syring, F.; Weigmann, H.J.; Schanzer, S.; Meinke, M.C.; Knorr, F.; Lademann, J. Investigation of Model Sunscreen Formulations Comparing the Sun Protection Factor, the Universal Sun Protection Factor and the Radical Formation Ratio. Skin Pharmacol. Physiol. 2016, 29, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.A.; Katiyar, S.K. Skin photoprotection by natural polyphenols: Anti-inflammatory, antioxidant and DNA repair mechanisms. Arch. Dermatol. Res. 2010, 302, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Andreo-Filho, N.; Bim, A.V.K.; Kaneko, T.M.; Kitice, N.A.; Haridass, I.N.; Abd, E.; Santos Lopes, P.; Thakur, S.S.; Parekh, H.S.; Roberts, M.S.; et al. Development and Evaluation of Lipid Nanoparticles Containing Natural Botanical Oil for Sun Protection: Characterization and in vitro and in vivo Human Skin Permeation and Toxicity. Skin Pharmacol. Physiol. 2018, 31, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sri Vidhya, M.; Bhanu Rekha, V. Effect of Knitted Bamboo Structures Dyed With Natural Colorants on Ultraviolet Radiation Protection. J. Text. Sci. Eng. 2012, 2, 2–4. [Google Scholar]
- Morrison, E.Y.; Thompson, H.; Pascoe, K.; West, M.; Fletcher, C. Extraction of an hyperglycaemic principle from the annatto (Bixa orellana), a medicinal plant in the West Indies. Trop. Geogr. Med. 1991, 43, 184–188. [Google Scholar] [PubMed]
- Mashmoul, M.; Azlan, A.; Khaza’ai, H.; Mohd Yusof, B.N.; Mohd Noor, S. Saffron: A Natural Potent Antioxidant as a Promising Anti-Obesity Drug. Antioxidants 2013, 2, 293–308. [Google Scholar] [CrossRef] [PubMed]
- Nair, S.C.; Pannikar, B.; Panikkar, K.R. Antitumour activity of saffron (Crocus sativus). Cancer Lett. 1991, 57, 109–114. [Google Scholar] [CrossRef]
- Ríos, J.L.; Recio, M.C.; Giner, R.M.; Máñez, S. An Update Review of Saffron and its Active Constituents. Phytother. Res. 1996, 10, 189–193. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Modaghegh, M.H.; Saffari, Z. Crocus Sativus L. (Saffron) Extract and its Active Constituents (Crocin and Safranal) on Ischemia-Reperfusion in Rat Skeletal Muscle. Evid.-Based Complement. Altern. Med. 2009, 6, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, H.; Younesi, H.M. Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacol. 2002, 2, 7. [Google Scholar]
- Chattopadhyay, S.N.; Pan, N.C.; Roy, A.K.; Saxena, S.; Khan, A. Development of natural dyed jute fabric with improved colour yield and UV protection characteristics. J. Text. Inst. 2013, 104, 808–818. [Google Scholar] [CrossRef]
- Golmohammadzadeh, S.; Jaafari, M.R.; Hosseinzadeh, H. Does Saffron Have Antisolar and Moisturizing Effects? Iran. J. Pharm. Res. 2010, 9, 133–140. [Google Scholar] [PubMed]
- Ing, L.Y.; Zin, N.M.; Sarwar, A.; Katas, H. Antifungal activity of chitosan nanoparticles and correlation with their physical properties. Int. J. Biomater. 2012, 2012, 632698. [Google Scholar] [CrossRef] [PubMed]
- Lee, F.Y.; Htar, T.T.; Akowuah, G.A. ATR-FTIR and Spectrometric Methods for the Assay of Crocin in Commercial Saffron Spices (Crocus savitus L.). Int. J. Food Prop. 2015, 18, 1773–1783. [Google Scholar] [CrossRef]
- Ioelovich, M. Crystallinity and Hydrophility of Chitin and Chitosan. J. Chem. 2014, 3, 7–14. [Google Scholar]
- Chrissafis, K.; Paraskevopoulos, K.M.; Papageorgiou, G.Z.; Bikiaris, D.N. Thermal and dynamic mechanical behavior of bionanocomposites: Fumed silica nanoparticles dispersed in poly(vinyl pyrrolidone), chitosan, and poly(vinyl alcohol). J. Appl. Polym. Sci. 2008, 110, 1739–1749. [Google Scholar] [CrossRef]
- Karavelidis, V.; Giliopoulos, D.; Karavas, E.; Bikiaris, D. Nanoencapsulation of a water soluble drug in biocompatible polyesters. Effect of polyesters melting point and glass transition temperature on drug release behavior. Eur. J. Pharm. Sci. 2010, 41, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Karavelidis, V.; Karavas, E.; Giliopoulos, D.; Papadimitriou, S.; Bikiaris, D. Evaluating the effects of crystallinity in new biocompatible polyester nanocarriers on drug release behavior. Int. J. Nanomed. 2011, 6, 3021–3032. [Google Scholar]
- Smaoui, S.; Ben Hlima, H.; Ben Chobba, I.; Kadri, A. Development and stability studies of sunscreen cream formulations containing three photo-protective filters. Arab. J. Chem. 2017, 10, S1216–S1222. [Google Scholar] [CrossRef]
- Some, I.T.; Bogaerts, P.; Hanus, R.; Hanocq, M.; Dubois, J. Improved kinetic parameter estimation in pH-profile data treatment. Int. J. Pharm. 2000, 198, 39–49. [Google Scholar] [CrossRef]
- Matousek, J.L.; Campbell, K.L.; Kakoma, I.; Solter, P.F.; Schaeffer, D.J. Evaluation of the effect of pH on in vitro growth of Malassezia pachydermatis. Can. J. Vet. Res. 2003, 67, 56–59. [Google Scholar] [PubMed]
- Nesseem, D. Formulation of sunscreens with enhancement sun protection factor response based on solid lipid nanoparticles. Int. J. Cosmet. Sci. 2011, 33, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Nasu, A.; Otsubo, Y. Effects of polymeric dispersants on the rheology and UV-protecting properties of complex suspensions of titanium dioxides and zinc oxides. Colloids Surfaces A Physicochem. Eng. Asp. 2008, 326, 92–97. [Google Scholar] [CrossRef]
- Calvo, P.; Vila-Jato, J.L.; Alonso, M.J. Evaluation of cationic polymer-coated nanocapsules as ocular drug carriers. Int. J. Pharm. 1997, 153, 41–50. [Google Scholar] [CrossRef]
- Aktas, Y.; Andrieux, K.; Alonso, M.J.; Calvo, P.; Gursoy, R.N.; Couvreur, P.; Capan, Y. Preparation and in vitro evaluation of chitosan nanoparticles containing a caspase inhibitor. Int. J. Pharm. 2005, 298, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Sayre, R.M.; Agin, P.P.; LeVee, G.J.; Marlowe, E. A comparison of in vivo and in vitro testing of sunscreening formulas. Photochem. Photobiol. 1979, 29, 559–566. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of all studied compounds are available from the authors. |








| Sample | D(50) (μm) | Zeta Potential (mV) | Yield (%) | Loading (%) | Entrapment Efficiency (%) |
|---|---|---|---|---|---|
| Blank | 250 ± 7 | 36 ± 3 | - | - | - |
| Annatto–CS | |||||
| 20 wt% | 287 ± 5 | 34 ± 3 | 70.83 | 15.62 | 66.40 |
| 40 wt% | 310 ± 8 | 41 ± 3 | 65.80 | 29.62 | 68.25 |
| 60 wt% | 340 ± 11 | 46 ± 3 | 61.25 | 41.17 | 67.25 |
| UF-annatto–CS | |||||
| 20 wt% | 263 ± 9 | 35 ± 3 | 47.80 | 15.69 | 45.01 |
| 40 wt% | 284 ± 10 | 40 ± 3 | 46.34 | 38.27 | 62.06 |
| 60 wt% | 303 ± 8 | 47 ± 3 | 55.07 | 48.71 | 71.54 |
| Saffron–CS | 321 ± 6 | 42 ± 3 | 67.75 | 20.52 | 21.25 |
| UF-saffron–CS | 298 ± 8 | 43 ± 3 | 83.75 | 25.61 | 37.50 |
| L | a* | b* | ||||
|---|---|---|---|---|---|---|
| Day 1 | Day 90 | Day 1 | Day 90 | Day 1 | Day 90 | |
| CS–Saffron | 89.58 | 89.17 | −0.34 | −0.37 | 2.77 | 2.68 |
| CS–UF-Saffron | 88.15 | 88.51 | −0.39 | −0.41 | 2.64 | 2.69 |
| CS–Annatto | 90.01 | 89.95 | −0.40 | −0.44 | 2.51 | 2.46 |
| CS–UF-Annatto | 91.11 | 91.18 | −0.42 | −0.45 | 2.49 | 2.51 |
| Sample | SPF Value |
|---|---|
| Blank | 1.00 |
| Annatto–CS | |
| 20 wt% | 2.63 |
| 40 wt% | 2.27 |
| 60 wt% | 2.24 |
| UF-annatto–CS | |
| 20 wt% | 2.76 |
| 40 wt% | 2.71 |
| 60 wt% | 2.56 |
| Saffron–CS | 2.15 |
| UF-saffron–CS | 4.85 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ntohogian, S.; Gavriliadou, V.; Christodoulou, E.; Nanaki, S.; Lykidou, S.; Naidis, P.; Mischopoulou, L.; Barmpalexis, P.; Nikolaidis, N.; Bikiaris, D.N. Chitosan Nanoparticles with Encapsulated Natural and UF-Purified Annatto and Saffron for the Preparation of UV Protective Cosmetic Emulsions. Molecules 2018, 23, 2107. https://doi.org/10.3390/molecules23092107
Ntohogian S, Gavriliadou V, Christodoulou E, Nanaki S, Lykidou S, Naidis P, Mischopoulou L, Barmpalexis P, Nikolaidis N, Bikiaris DN. Chitosan Nanoparticles with Encapsulated Natural and UF-Purified Annatto and Saffron for the Preparation of UV Protective Cosmetic Emulsions. Molecules. 2018; 23(9):2107. https://doi.org/10.3390/molecules23092107
Chicago/Turabian StyleNtohogian, Sonia, Viktoria Gavriliadou, Evi Christodoulou, Stavroula Nanaki, Smaro Lykidou, Panagiotis Naidis, Lily Mischopoulou, Panagiotis Barmpalexis, Nikolaos Nikolaidis, and Dimitrios N. Bikiaris. 2018. "Chitosan Nanoparticles with Encapsulated Natural and UF-Purified Annatto and Saffron for the Preparation of UV Protective Cosmetic Emulsions" Molecules 23, no. 9: 2107. https://doi.org/10.3390/molecules23092107
APA StyleNtohogian, S., Gavriliadou, V., Christodoulou, E., Nanaki, S., Lykidou, S., Naidis, P., Mischopoulou, L., Barmpalexis, P., Nikolaidis, N., & Bikiaris, D. N. (2018). Chitosan Nanoparticles with Encapsulated Natural and UF-Purified Annatto and Saffron for the Preparation of UV Protective Cosmetic Emulsions. Molecules, 23(9), 2107. https://doi.org/10.3390/molecules23092107