Comparative Phytochemical Profiles and Antioxidant Enzyme Activity Analyses of the Southern Highbush Blueberry (Vaccinium corymbosum) at Different Developmental Stages
Abstract
:1. Introduction
2. Results and Discussion
2.1. Growth Data and Water Content of Different Developmental Stages of Fruit
2.2. Influence of Different Developmental Stages of Fruit on Soluble Sugars (SS) and Titratable Acidity (TA)
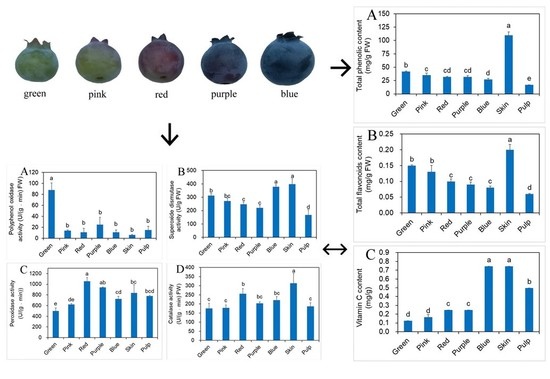
2.3. Effects of Different Developmental Stages of Fruit on Phenolic, Flavonoids and vit. C Contents
2.4. Total Anthocyanin Content (TAC) and Qualitative Identification by HPLC
2.5. Influence of Different Developmental Stages of Fruit on Antioxidant Enzyme Activity
3. Materials and Methods
3.1. Plant Materials
3.2. Instruments and Chemicals
3.3. Growth Data and Water Content
3.4. Soluble Sugars (SS)
3.5. Titratable Acidity (TA)
3.6. Total Phenolic Content (TPC)
3.7. Total Flavonoids Content (TFC)
3.8. Total Anthocyanin Content (TAC)
3.9. Vitamin C content (vit. C)
3.10. Anthocyanin Qualitative Identification by HPLC
3.11. Polyphenol Oxidase Activity (PPO)
3.12. Superoxide Dismutase Activity (SOD)
3.13. Peroxidase Activity (POD)
3.14. Catalase Activity (CAT)
3.15. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ouerghemmi, I.; Bettaieb, I.R.; Rahali, F.Z.; Bourgou, S.; Pistelli, L.; Ksouri, R.; Marzouk, B.; Saidani, M.T. Antioxidant and antimicrobial phenolic compounds from extracts of cultivated and wild-grown tunisian ruta chalepensis. J. Food Drug Anal. 2017, 25, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Li, G.; Long, C.; Xu, J.; Cen, J.; Yang, X. The antioxidant activity and genotoxicity of isogarcinol. Food Chem. 2018, 253, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Siddiq, M.; Dolan, K.D. Characterization of polyphenol oxidase from blueberry (Vaccinium corymbosum L.). Food Chem. 2017, 218, 216–220. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Guo, X.; Hu, X.; Li, T.; Fu, X.; Liu, R.H. Comparison of phytochemical profiles, antioxidant and cellular antioxidant activities of different varieties of blueberry (Vaccinium spp.). Food Chem. 2017, 217, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Diaconeasa, Z.; Leopold, L.; Rugină, D.; Ayvaz, H.; Socaciu, C. Antiproliferative and antioxidant properties of anthocyanin rich extracts from blueberry and blackcurrant juice. Int. J. Mol. Sci. 2015, 16, 2352–2365. [Google Scholar] [CrossRef] [PubMed]
- Taverniti, V.; Fracassetti, D.; Del Bo’, C.; Lanti, C.; Minuzzo, M.; Klimiszacas, D.; Riso, P.; Guglielmetti, S. Immunomodulatory effect of a wild blueberry anthocyanin-rich extract in human caco-2 intestinal cells. J. Agric. Food Chem. 2014, 62, 8346–8351. [Google Scholar] [CrossRef] [PubMed]
- Martineau, L.C.; Couture, A.; Spoor, D.; Benhaddouandaloussi, A.; Harris, C.; Meddah, B.; Leduc, C.; Burt, A.; Vuong, T.; Mai, L.P. Anti-diabetic properties of the canadian lowbush blueberry vaccinium angustifolium ait. Phytomedicine 2006, 13, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Giovanelli, G.; Buratti, S. Comparison of polyphenolic composition and antioxidant activity of wild Italian blueberries and some cultivated varieties. Food Chem. 2009, 112, 903–908. [Google Scholar] [CrossRef]
- De Souza, V.R.; Pereira, P.A.P.; da Silva, T.L.T.; de Oliveira Lima, L.C.; Pio, R.; Queiroz, F. Determination of the bioactive compounds, antioxidant activity and chemical composition of brazilian blackberry, red raspberry, strawberry, blueberry and sweet cherry fruits. Food Chem. 2014, 156, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Intuyod, K.; Priprem, A.; Limphirat, W.; Charoensuk, L.; Pinlaor, P.; Pairojkul, C.; Lertrat, K.; Pinlaor, S. Anti-inflammatory and anti-periductal fibrosis effects of an anthocyanin complex in opisthorchis viverrini-infected hamsters. Food Chem. Toxicol. 2014, 74, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Anttonen, M.J.; Karjalainen, R.O. Environmental and genetic variation of phenolic compounds in red raspberry. J. Food Comp. Anal. 2005, 18, 759–769. [Google Scholar] [CrossRef]
- Wang, S.Y.; Chen, H.; Ehlenfeldt, M.K. Variation in antioxidant enzyme activities and nonenzyme components among cultivars of rabbiteye blueberries (Vaccinium ashei Reade) and v. Ashei derivatives. Food Chem. 2011, 129, 13–20. [Google Scholar] [CrossRef]
- Cervantes-Elizarrarás, A.; Piloni-Martini, J.; Ramírez-Moreno, E.; Alanís-García, E.; Güemes-Vera, N.; Gómez-Aldapa, C.A.; Zafra-Rojas, Q.Y.; Cruz-Cansino, N.D.S. Enzymatic inactivation and antioxidant properties of blackberry juice after thermoultrasound: Optimization using response surface methodology. Ultrason. Sonochem. 2017, 34, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Cezarotto, V.S.; Giacomelli, S.R.; Vendruscolo, M.H.; Vestena, A.S.; Cezarotto, C.S.; Da, R.C.; Maurer, L.H.; Ferreira, L.M.; Emanuelli, T.; Cruz, L. Influence of harvest season and cultivar on the variation of phenolic compounds composition and antioxidant properties in vaccinium ashei leaves. Molecules 2017, 22, 1603. [Google Scholar] [CrossRef] [PubMed]
- Zadernowski, R.; Naczk, M.; Nesterowicz, J. Phenolic acid profiles in some small berries. J. Agric. Food Chem. 2005, 53, 2118–2124. [Google Scholar] [CrossRef] [PubMed]
- Zielinska, M.; Michalska, A. Microwave-assisted drying of blueberry (Vaccinium corymbosum L.) fruits: Drying kinetics, polyphenols, anthocyanins, antioxidant capacity, colour and texture. Food Chem. 2016, 212, 671–680. [Google Scholar] [CrossRef] [PubMed]
- Mahmood, T.; Anwar, F.; Abbas, M.; Boyce, M.C.; Saari, N. Compositional variation in sugars and organic acids at different maturity stages in selected small fruits from pakistan. Int. J. Mol. Sci. 2012, 13, 1380–1392. [Google Scholar] [CrossRef] [PubMed]
- Serradilla, M.J.; Lozano, M.; Bernalte, M.J.; Ayuso, M.C.; Lópezcorrales, M.; Gonzálezgómez, D. Physicochemical and bioactive properties evolution during ripening of ‘ambrunés’ sweet cherry cultivar. LWT Food Sci. Technol. 2011, 44, 199–205. [Google Scholar] [CrossRef]
- Bates, R.P.; Morris, J.R.; Crandall, P.G. Principles and Practices of Small- and Medium-Scale Fruit Juice Processing; Fao Agricultural Services Bulletin: Rome, Italy, 2001. [Google Scholar]
- Eichholz, I.; Huyskens-Keil, S.; Rohn, S. Chapter 21—blueberry phenolic compounds: Fruit maturation, ripening and post-harvest effects. Process. Impact Act. Compon. Food 2015, 173–180. [Google Scholar] [CrossRef]
- Castrejón, A.D.R.; Eichholz, I.; Rohn, S.; Kroh, L.W.; Huyskens-Keil, S. Phenolic profile and antioxidant activity of highbush blueberry (Vaccinium corymbosum L.) during fruit maturation and ripening. Food Chem. 2008, 109, 564–572. [Google Scholar] [CrossRef]
- Kalt, W.; Ryan, D.A.; Duy, J.C.; Prior, R.L.; Ehlenfeldt, M.K.; Vander Kloet, S.P. Interspecific variation in anthocyanins, phenolics, and antioxidant capacity among genotypes of highbush and lowbush blueberries (Vaccinium section cyanococcus spp.). J. Agric. Food Chem. 2001, 49, 4761–4767. [Google Scholar] [CrossRef] [PubMed]
- Nogata, Y.; Sakamoto, K.; Shiratsuchi, H.; Ishii, T.; Yano, M.; Ohta, H. Flavonoid composition of fruit tissues of citrus species. Biosci. Biotechnol. Biochem. 2006, 70, 178–192. [Google Scholar] [CrossRef] [PubMed]
- Shwartz, E.; Glazer, I.; Bar-Ya’Akov, I.; Matityahu, I.; Bar-Ilan, I.; Holland, D.; Amir, R. Changes in chemical constituents during the maturation and ripening of two commercially important pomegranate accessions. Food Chem. 2009, 115, 965–973. [Google Scholar] [CrossRef]
- Jaakola, L.; Hohtola, A. Expression of genes involved in anthocyanin biosynthesis in relation to anthocyanin, proanthocyanidin, and flavonol levels during bilberry fruit development. Plant Physiol. 2002, 130, 729–739. [Google Scholar] [CrossRef] [PubMed]
- Halbwirth, H.; Puhl, I.; Haas, U.; Jezik, K.; Treutter, D.; Stich, K. Two-phase flavonoid formation in developing strawberry (fragaria × ananassa) fruit. J Agric. Food Chem. 2006, 54, 1479–1485. [Google Scholar] [CrossRef] [PubMed]
- Jing, P.; Noriega, V.; Schwartz, S.J.; Giusti, M.M. Effects of growing conditions on purple corncob (Zea mays L.) anthocyanins. J. Agric. Food Chem. 2007, 55, 8625–8629. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Johnsoncicalese, J.; Singh, A.P.; Vorsa, N. Characterization and quantification of flavonoids and organic acids over fruit development in american cranberry (Vaccinium macrocarpon) cultivars using hplc and apci-ms/ms. Plant Sci. 2017, 262, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Grace, M.H.; Ribnicky, D.M.; Kuhn, P.; Poulev, A.; Logendra, S.; Yousef, G.G.; Raskin, I.; Lila, M.A. Hypoglycemic activity of a novel anthocyanin-rich formulation from lowbush blueberry, vaccinium angustifolium aiton. Phytomedicine 2009, 16, 406–415. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Xu, J. Profiles of carotenoids, anthocyanins, phenolics, and antioxidant activity of selected color waxy corn grains during maturation. J. Agric. Food Chem. 2011, 59, 2026–2033. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Chen, J.; Yang, H.; Chen, W.; Gao, H.; Lu, W. Variation in total anthocyanin, phenolic contents, antioxidant enzyme and antioxidant capacity among different mulberry (Morus sp.) cultivars in china. Sci. Hortic. 2016, 213, 186–192. [Google Scholar] [CrossRef]
- Böttcher, H.; Günther, I.; Kabelitz, L. Physiological postharvest responses of common saint-john’s wort herbs (Hypericum perforatum L.). Postharvest Biol. Technol. 2003, 29, 343–351. [Google Scholar] [CrossRef]
- Moyer, R.A.; Hummer, K.E.; Finn, C.E.; Frei, B.; Wrolstad, R.E. Anthocyanins, phenolics, and antioxidant capacity in diverse small fruits: Vaccinium, rubus, and ribes. J. Agric. Food Chem. 2002, 50, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Kalt, W.; Lawand, C.; Ryan, D.A.J.; Mcdonald, J.E.; Donner, H.; Forney, C.F. Oxygen radical absorbing capacity, anthocyanin and phenolic content of highbush blueberries (Vaccinium corymbosum L.) during ripening and storage. J. Am. Soc. Hortic. Sci. 2003, 128, 917–923. [Google Scholar]
- Mohammadi, M.; Kazemi, H. Changes in peroxidase and polyphenol oxidase activities in susceptible and resistant wheat heads inoculated with fusarium graminearum and induced resistance. Plant Sci. 2002, 162, 491–498. [Google Scholar] [CrossRef]
- Chittoor, J.M. Induction of Peroxidase during Defense against Pathogens; CRC: Boca Raton, FL, USA, 1999. [Google Scholar]
- Zhu, G.; Zhong, H.; Zhang, A. Plant Physiological Experiment; Peking University Press: Beijing, China, 1990. [Google Scholar]
- Wang, X.; Huang, J. Experimental Principle and Technology of Plant Physiology and Biochemistry; Higher Education Press: Beijing, China, 2015. [Google Scholar]
- Pastrana-Bonilla, E.; Akoh, C.C.; Sellappan, S.; Krewer, G. Phenolic content and antioxidant capacity of muscadine grapes. J. Agric. Food Chem. 2003, 51, 5497–5503. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Gonzalez, E. Polyphenolic compounds, antioxidant capacity, and quinone reductase activity of an aqueous extract of ardisia compressa in comparison to mate (ilex paraguariensis) and green (camellia sinensis) teas. J. Agric. Food Chem. 2004, 52, 3583–3589. [Google Scholar] [CrossRef] [PubMed]
- Škerget, M.; Kotnik, P.; Hadolin, M.; Hraš, A.R.; Simonič, M.; Knez, Ž. Phenols, proanthocyanidins, flavones and flavonols in some plant materials and their antioxidant activities. Food Chem. 2005, 89, 191–198. [Google Scholar] [CrossRef]
- Wolfe, K.; Wu, X.; Liu, R. Antioxidant activity of apple peels. J. Agric. Food Chem. 2003, 51, 609–614. [Google Scholar] [CrossRef] [PubMed]
- Fuleki, T.; Francis, F.J. Quantitative methods for anthocyanins. 1. Extraction and determination of total anthocyanin in cranberries. J. Food Sci. 1968, 33, 72–77. [Google Scholar] [CrossRef]
- Rao, M.V.; Paliyath, G.; Ormrod, D.P. Ultraviolet-b- and ozone-induced biochemical changes in antioxidant enzymes of arabidopsis thaliana. Plant Physiol. 1996, 110, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Kochba, J.; Lavee, S.; SpiegelRoy, P. Differences in peroxidase activity and isoenzymes in embryogenic ane non-embryogenic ‘shamouti’ orange ovular callus lines. Plant Cell Physiol. 1977, 18, 463–467. [Google Scholar] [CrossRef]
- Verma, S.; Mishra, S.N. Putrescine alleviation of growth in salt stressed brassica juncea by inducing antioxidative defense system. J. Plant Physiol. 2005, 162, 669–677. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the compounds are not available from the authors. |






| Growth Stages | Transverse Diameter (cm) | Vertical Diameter (cm) | Fruit Shape Index | Fruit Weight (g) | Dry Matter (%) | Water Content (%) |
|---|---|---|---|---|---|---|
| Green | 0.97 | 0.98 | 1.01 | 0.43 | 14.95 | 85.05 |
| Pink | 0.98 | 0.98 | 1.00 | 0.63 | 14.36 | 85.64 |
| Red | 1.13 | 1.03 | 0.91 | 0.67 | 13.95 | 86.05 |
| Purple | 1.13 | 1.13 | 1.00 | 0.77 | 14.29 | 85.71 |
| Blue | 1.29 | 1.20 | 0.93 | 1.11 | 15.06 | 84.94 |
| Skin | -- | -- | -- | -- | 15.19 | 84.81 |
| Pulp | -- | -- | -- | -- | 15.15 | 84.85 |
| Peak No. | TR (min) a | Identification | Reference b |
|---|---|---|---|
| 1 | 24.92 | Delphinidin-3-glucoside | Standard |
| 2 | 26.17 | Delphinidin-3-arabinoside | Grace M H et al. (2009) |
| 3 | 27.14 | Cyanidin-3-galactoside | Grace M H et al. (2009) |
| 4 | 28.16 | Cyanidin-3-arabinoside | Grace M H et al. (2009) |
| 5 | 29.49 | Cyanidin-3-glucoside | Standard |
| 6 | 30.30 | Peonidin-3-galactoside | Grace M H et al. (2009) |
| 7 | 31.06 | Petunidin-3-glucoside | Standard |
| 8 | 33.73 | Peonidin-3-glucoside | Standard |
| 9 | 34.33 | Malvidin-3-galactoside | Grace M H et al. (2009) |
| 10 | 35.15 | Malvidin-3-glucoside | Standard |
| 11 | 36.30 | Malvidin-3-arabinoside | Grace M H et al. (2009) |
| 12 | 36.65 | Delphenidin-6-acetyl-3-glucoside | Grace M H et al. (2009) |
| TPC | TFC | TAC | vit. C | SOD | POD | PPO | CAT | |
|---|---|---|---|---|---|---|---|---|
| TPC | 1.00 | |||||||
| TFC | 0.91 ** | 1.00 | ||||||
| TAC | 0.93 ** | 0.71 ** | 1.00 | |||||
| vit. C | 0.41 | 0.09 | 0.70 ** | 1.00 | ||||
| SOD | 0.63 ** | 0.63 ** | 0.65 ** | 0.50 * | 1.00 | |||
| POD | 0.02 | 0.24 a | 0.16 | 0.17 | 0.21 a | 1.00 | - | |
| PPO | 0.11 a | 0.17 | 0.34 a | 0.54 a,* | 0.01 a | 0.60 a,** | 1.00 | |
| CAT | 0.72 ** | 0.53 * | 0.78 ** | 0.55 * | 0.44 * | 0.42 | 0.45 a,* | 1.00 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Li, M.; Mitra, S.; Hafiz Muhammad, R.; Debnath, B.; Lu, X.; Jian, H.; Qiu, D. Comparative Phytochemical Profiles and Antioxidant Enzyme Activity Analyses of the Southern Highbush Blueberry (Vaccinium corymbosum) at Different Developmental Stages. Molecules 2018, 23, 2209. https://doi.org/10.3390/molecules23092209
Sun Y, Li M, Mitra S, Hafiz Muhammad R, Debnath B, Lu X, Jian H, Qiu D. Comparative Phytochemical Profiles and Antioxidant Enzyme Activity Analyses of the Southern Highbush Blueberry (Vaccinium corymbosum) at Different Developmental Stages. Molecules. 2018; 23(9):2209. https://doi.org/10.3390/molecules23092209
Chicago/Turabian StyleSun, Yueting, Min Li, Sangeeta Mitra, Rizwan Hafiz Muhammad, Biswojit Debnath, Xiaocao Lu, Hongxiang Jian, and Dongliang Qiu. 2018. "Comparative Phytochemical Profiles and Antioxidant Enzyme Activity Analyses of the Southern Highbush Blueberry (Vaccinium corymbosum) at Different Developmental Stages" Molecules 23, no. 9: 2209. https://doi.org/10.3390/molecules23092209
APA StyleSun, Y., Li, M., Mitra, S., Hafiz Muhammad, R., Debnath, B., Lu, X., Jian, H., & Qiu, D. (2018). Comparative Phytochemical Profiles and Antioxidant Enzyme Activity Analyses of the Southern Highbush Blueberry (Vaccinium corymbosum) at Different Developmental Stages. Molecules, 23(9), 2209. https://doi.org/10.3390/molecules23092209