One-pot Fluorination and Organocatalytic Robinson Annulation for Asymmetric Synthesis of Mono- and Difluorinated Cyclohexenones
Abstract
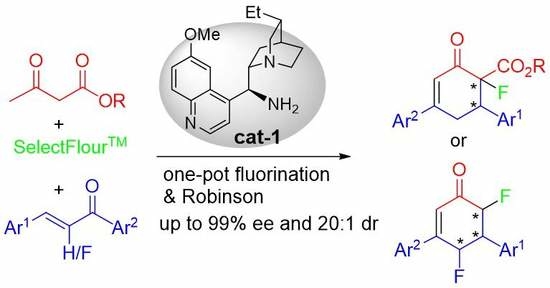
:1. Introduction
2. Results and Discussions
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mikami, K.; Itoh, Y.; Yamanaka, M. Fluorinated carbonyl and olefinic compounds: Basic character and asymmetric catalytic reactions. Chem. Rev. 2004, 104, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Smits, R.; Cadicamo, C.D.; Burger, K.; Koksch, B. Synthetic strategies to α-trifluoromethyl and α-difluoromethyl substituted α-amino acids. Chem. Soc. Rev. 2008, 37, 1727–1739. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Sánchez-Roselló, M.; Acena, J.L.; del Pozo, C.; So-rochinsky, A.E.; Fustero, S.; Soloshonok, V.A.; Liu, H. Fluorine in pharmaceutical industry: Fluorine-containing drugs introduced to the market in the last decade (2001–2011). Chem. Rev. 2014, 114, 2432–2506. [Google Scholar] [CrossRef] [PubMed]
- Ojima, I. Fluorine in Medicinal Chemistry and Chemical Biology; Wiley-Blackwell: Chichester, UK, 2009. [Google Scholar]
- Ho, T.L. Carbocycle Construction in Terpene Synthesis; Wiley-VCH: Weinheim, Germany, 1988. [Google Scholar]
- Paolo, P.; Manuela, V.; Mario, V.; Antonella, I. 4,5,6,7-Tetrahydroindazole Derivatives as Antitumor Agents. WO Patent 2,000,069,846, 23 December 2000. [Google Scholar]
- Roy, P.P.; Roy, K. Molecular docking and QSAR studies of aromatase inhibitor androstenedione derivatives. J. Pharm. Pharmacol. 2010, 62, 1717–1728. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shelton, N.L.; Liu, R.S.H. Study of α-crustacyanin utilizing halogenated canthaxanthins. Org. Lett. 2002, 4, 2521–2524. [Google Scholar] [CrossRef] [PubMed]
- Govender, T.; Arvidsson, P.I.; Maguire, G.E.M.; Kruger, H.G.; Naicker, T. Enantioselective organocatalyzed transformations of β-Ketoesters. Chem. Rev. 2016, 116, 9375–9437. [Google Scholar] [CrossRef] [PubMed]
- Oh, Y.; Kim, S.M.; Kim, D.Y. Organocatalytic synthesis of quaternary stereocenter bearing a fluorine atom: Enantioselective conjugate addition of α-fluoro-β-ketoesters to nitroalkenes. Tetrahedron Lett. 2009, 50, 4674–4676. [Google Scholar] [CrossRef]
- Lu, Y.; Zou, G.; Zhao, G. Primary-secondary diamines catalyzed Michael reaction to generate chiral fluorinated quaternary carbon centers. Tetrahedron 2015, 71, 4137–4144. [Google Scholar] [CrossRef]
- Huang, X.; Yi, W.B.; Ahad, D.; Zhang, W. Recyclable cinchona alkaloid catalyzed asymmetric Michael addition reaction. Tetrahedron Lett. 2013, 54, 6064–6066. [Google Scholar] [CrossRef]
- Han, Y.; Luo, J.; Liu, C.; Lu, Y. Asymmetric generation of fluorine-containing quaternary carbons adjacent to tertiary stereocenters: Uses of fluorinated methines as nucleophiles. Chem. Commun. 2009, 0, 2044–2046. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, S.; Yu, C.; Song, X.; Wang, W. Organocatalytic asymmetric synthesis of chiral fluorinated quaternary carbon containing β-ketoesters. Chem. Commun. 2009, 0, 2136–2138. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Pan, Y.; Zhao, Y.; Ma, T.; Lee, R.; Yang, Y.; Huang, K.W.; Wong, M.W.; Tan, C.H. Synthesis of a chiral quaternary carbon center bearing a fluorine atom: Enantio- and diastereoselective guanidine-catalyzed addition of fluorocarbon nucleophiles. Angew. Chem. Int. Ed. 2009, 48, 3627–3631. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.-F.; Li, P.; Wang, X.-W.; Zhu, S.-Z.; Zhao, G. Asymmetric Michael addition of a-fluoro-a-phenylsulfonyl ketones to nitroolefins catalyzed by phenylalanine-based bifunctional thioureas. J. Fluorine Chem. 2012, 133, 120–126. [Google Scholar] [CrossRef]
- Prakash, G.K.S.; Wang, F.; Stewart, T.; Mathew, T.; Olah, G.A. α-Fluoro-β-nitro(phenylsulfonyl)methane as a fluoromethyl pronucleophile: Efficient stereoselective Michael addition to chalcones. Proc. Natl. Acad. Sci. USA. 2009, 106, 4090–4094. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, A.; Shibata, N.; Mizuta, S.; Nakamura, S.; Toru, T.; Shiro, M. Catalytic enantioselective Michael addition of 1-fluorobis(phenylsulfonyl)methane to α,β-unsaturated ketones catalyzed by cinchona alkaloids. Angew. Chem. Int. Ed. 2008, 47, 8051–8054. [Google Scholar] [CrossRef] [PubMed]
- Ullah, F.; Zhao, G.L.; Deiana, L.; Zhu, M.; Dziedzic, P.; Ibrahem, I.; Hammar, P.; Sun, J.; Córdova, A. Enantioselective organocatalytic conjugate addition of fluorocarbon nucleophiles to α,β-unsaturated aldehydes. Chem. Eur. J. 2009, 15, 10013–10017. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.F.; Yang, Y.Q.; Chai, Z.; Li, P.; Zheng, C.W.; Zhu, S.Z.; Zhao, G. Enantioselective synthesis of functionalized fluorinated cyclohexenones via Robinson annulation catalyzed by primary−secondary diamines. J. Org. Chem. 2010, 75, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Liu, M.; Jasinski, J.P.; Peng, B.; Zhang, W. Recyclable organocatalysts for a one-pot asymmetric synthesis of 2-fluorocyclohexanols bearing six contiguous stereocenters. Adv. Synth. Catal. 2017, 359, 1919–1926. [Google Scholar] [CrossRef]
- Huang, X.; Pham, K.; Zhang, X.-F.; Yi, W.-B.; Hyatt, J.; Tran, A.; Jasinski, J.P.; Zhang, W. Recyclable organocatalyst for one-pot asymmetric synthesis of fluorinated 2-Piperidinones bearing four contiguous stereogenic centers. RSC Adv. 2015, 5, 71071–71075. [Google Scholar] [CrossRef]
- Yi, W.B.; Huang, X.; Cai, C.; Zhang, W. One-pot fluorination followed by Michael addition or Robinson annulation for preparation of α-fluorinated carbonyl compounds. Green Chem. 2012, 14, 3185–3189. [Google Scholar] [CrossRef]
- Qian, J.; Yi, W.B.; Huang, X.; Miao, Y.; Zhang, J.; Cai, C.; Zhang, W. One-pot synthesis of 3,5-disubstituted and polysubstituted phenols from acyclic precursors. Org. Lett. 2015, 17, 1090–1093. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Huang, X.; Yi, W.B.; Zhang, W. One-pot reactions for modular synthesis of polysubstituted and fused pyridines. Org. Lett. 2016, 18, 5640–5643. [Google Scholar] [CrossRef] [PubMed]
- Brazier, J.B.; Tomkinson, N.C.O. Secondary and primary amine catalysts for iminium catalysis. In Asymmetric Organocatalysis; List, B., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; Volume 291, pp. 281–347. [Google Scholar]
- Halland, N.; Aburel, P.S.; Jørgensen, K.A. Highly enantio- and diastereoselective organocatalytic asymmetric domino Michael–aldol reaction of β-ketoesters and α,β-unsaturated ketones. Angew. Chem. Int. Ed. 2004, 43, 1272–1277. [Google Scholar]
Sample Availability: Samples of the compounds are available from the authors. |





| Entry | Cat | Additive | Yield (%) b | dr c | ee (%) d |
|---|---|---|---|---|---|
| 1 | cat-1 | C6H5CO2H | 79 | 5:1 | 99 |
| 2 | cat-2 | C6H5CO2H | NR | - | - |
| 3 | cat-3 | C6H5CO2H | NR | - | - |
| 4 | cat-4 | C6H5CO2H | NR | - | - |
| 5 | cat-5 | C6H5CO2H | trace | - | - |
| 6 | cat-6 | C6H5CO2H | 27 | 3:1 | 85 |
| 7 | cat-1 | None | 42 | 5:1 | 92 |
| 8 | cat-1 | AcOH | 52 | 6:1 | 92 |
| 9 | cat-1 | TsOH | 45 | 5:1 | 95 |
| 10 | cat-1 | CF3C6H4CO2H | 89 | 9:1 | 99 |
| 11 | cat-1 | CCl3CO2H | 75 | 8:1 | 90 |
| 12 | cat-1 | CF3CO2H | 77 | 8:1 | 90 |
| 13e | cat-1 | CF3C6H4CO2H | 51 | 12:1 | 99 |

| Entry | Base (equiv) | Yield (%) b | dr c | ee (%) d |
|---|---|---|---|---|
| 1 | None | <10 | - | - |
| 2 | Na2CO3 (0.5) | 42 | 9:1 | 99 |
| 3 | K2CO3 (0.5) | 67 | 3:1 | 85 |
| 4 | Cs2CO3(0.5) | 85 | 1.5:1 | 52 |
| 5 | Na2CO3 (1.0) | 57 | 9:1 | 99 |
| 6 | Na2CO3 (1.5) | 82 | 9:1 | 99 |
| 7 | Na2CO3 (2.0) | 83 | 9:1 | 99 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, X.; Zhao, W.; Zhang, X.; Liu, M.; Vasconcelos, S.N.S.; Zhang, W. One-pot Fluorination and Organocatalytic Robinson Annulation for Asymmetric Synthesis of Mono- and Difluorinated Cyclohexenones. Molecules 2018, 23, 2251. https://doi.org/10.3390/molecules23092251
Huang X, Zhao W, Zhang X, Liu M, Vasconcelos SNS, Zhang W. One-pot Fluorination and Organocatalytic Robinson Annulation for Asymmetric Synthesis of Mono- and Difluorinated Cyclohexenones. Molecules. 2018; 23(9):2251. https://doi.org/10.3390/molecules23092251
Chicago/Turabian StyleHuang, Xin, Weizhao Zhao, Xiaofeng Zhang, Miao Liu, Stanley N. S. Vasconcelos, and Wei Zhang. 2018. "One-pot Fluorination and Organocatalytic Robinson Annulation for Asymmetric Synthesis of Mono- and Difluorinated Cyclohexenones" Molecules 23, no. 9: 2251. https://doi.org/10.3390/molecules23092251
APA StyleHuang, X., Zhao, W., Zhang, X., Liu, M., Vasconcelos, S. N. S., & Zhang, W. (2018). One-pot Fluorination and Organocatalytic Robinson Annulation for Asymmetric Synthesis of Mono- and Difluorinated Cyclohexenones. Molecules, 23(9), 2251. https://doi.org/10.3390/molecules23092251