Triterpenoids from Cyclocarya paliurus that Enhance Glucose Uptake in 3T3-L1 Adipocytes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Identification of New Compounds
2.2. Glucose Uptake Assay
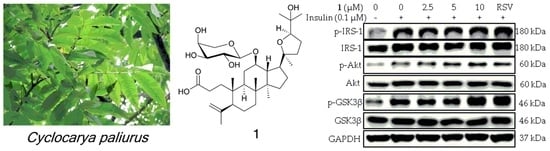
2.3. Compound 1 Enhances Insulin Sensitivity in 3T3-L1 Adipocytes through Activating AMP-Activated Protein Kinase (AMPK)-p38 Pathway
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation
3.4. Acid Hydrolysis of Compounds 1–3
3.5. Reagents
3.6. Cell Culture and Treatment
3.7. Cell Viability Assay
3.8. Glucose Uptake Assay
3.9. Western Blot Analysis
3.10. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mahomoodally, M.F.; Mootoosamy, A.; Wambugu, S. Traditional therapies used to manage diabetes and related complications in mauritius: A comparative ethnoreligious study. J. Evid.-Based Complement. Altern. Med. 2016, 2016, 4523828. [Google Scholar] [CrossRef] [PubMed]
- Maruthur, N.M.; Tseng, E.; Hutfless, S.; Wilson, L.M.; Suarez-Cuervo, C.; Berger, Z.; Chu, Y.; Iyoha, E.; Segal, J.B.; Bolen, S. Diabetes medications as monotherapy or metformin-based combination therapy for Type 2 Diabetes: A systematic review and meta-analysis. Ann. Intern. Med. 2016, 164, 740–751. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, D.; Singh, V.; Gilani, S.J.; Goel, R.; Grover, P.; Vats, A. Hypoglycemic herbs and their polyherbal formulations: A comprehensive review. Med. Chem. Res. 2015, 24, 1–21. [Google Scholar] [CrossRef]
- Han, J.H.; Tuan, N.Q.; Park, M.H.; Quan, K.T.; Oh, J.; Heo, K.S.; Na, M.; Myung, C.S. Cucurbitane triterpenoids from the fruits of Momordica Charantia improve insulin sensitivity and glucose homeostasis in streptozotocin-induced diabetic mice. Mol. Nutr. Food Res. 2018, 62, 1700769. [Google Scholar] [CrossRef] [PubMed]
- Sudirman, S.; Hsu, Y.H.; Kong, Z.L. The amelioration effects of nanoencapsulated triterpenoids from petri dish-cultured Antrodia cinnamomea on reproductive function of diabetic male rats. Int. J. Chem. Biol. Eng. 2018, 12, 5059–5073. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, T.; Li, Y.; Yuan, C.; Ma, H.; Seeram, N.P.; Liu, F.; Mu, Y.; Huang, X.; Li, L. Hypoglycemic and hypolipidemic effects of triterpenoid-enriched Jamun (Eugenia jambolana Lam.) fruit extract in streptozotocin-induced type 1 diabetic mice. Food Funct. 2018, 9, 3330–3337. [Google Scholar] [CrossRef] [PubMed]
- Rao, V.S.; de Melo, C.L.; Queiroz, M.G.R.; Lemos, T.L.G.; Menezes, D.B.; Melo, T.S.; Santos, F.A. Ursolic acid, a pentacyclic triterpene from Sambucus australis, prevents abdominal adiposity in mice fed a high-fat diet. J. Med. Food 2011, 14, 1375–1382. [Google Scholar] [CrossRef]
- Kazmi, I.; Rahman, M.; Afzal, M.; Gupta, G.; Saleem, S.; Afzal, O.; Shaharyar, M.A.; Nautiyal, U.; Ahmed, S.; Anwar, F. Anti-diabetic potential of ursolic acid stearoyl glucoside: A new triterpenic gycosidic ester from Lantana camara. Fitoterapia 2012, 83, 142–146. [Google Scholar] [CrossRef]
- Editor Committee for Flora of China of Chinese Academy of Science. Flora of China; Science Publishing House: Beijing, China, 1979; Volume 21, pp. 18–19. [Google Scholar]
- The Editorial Committee of the Administration Bureau of Traditional Chinese Medicine. Chinese Materia Medica (ZhonghuaBencao); Shanghai Science and Technology Publishing House: Shanghai, China, 1999; Volume 2, pp. 370–371. [Google Scholar]
- Li, S.; Li, J.; Guan, X.L.; Li, J.; Deng, S.P.; Li, L.Q.; Tang, M.T.; Huang, J.G.; Chen, Z.Z.; Yang, R.Y. Hypoglycemic effects and constituents of the barks of Cyclocarya paliurus and their inhibiting activities to glucosidase and glycogen phosphorylase. Fitoterapia 2011, 82, 1081–1085. [Google Scholar] [CrossRef]
- Xie, J.H.; Xie, M.Y.; Nie, S.P.; Shen, M.Y.; Wang, Y.X.; Li, C. Isolation, chemical composition and antioxidant activities of a water-soluble polysaccharide from Cyclocarya paliurus (Batal.) Iljinskaja. Food Chem. 2010, 119, 1626–1632. [Google Scholar] [CrossRef]
- Zhang, J.; Shen, Q.; Lu, J.C.; Li, J.Y.; Liu, W.Y.; Yang, J.J.; Li, J.; Xiao, K. Phenolic compounds from the leaves of Cyclocarya paliurus (Batal.) Ijinskaja and their inhibitory activity against PTP1B. Food Chem. 2010, 119, 1491–1496. [Google Scholar] [CrossRef]
- Li, S.; Cui, B.S.; Liu, Q.; Tang, L.; Yang, Y.C.; Jin, X.J.; Shen, Z.F. New triterpenoids from the leaves of Cyclocarya paliurus. Planta Med. 2012, 78, 290–296. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Jiang, C.; Fang, S.; Wang, J.; Ji, Y.; Shang, X.; Ni, Y.; Yin, Z.; Zhang, J. Antihyperglycemic, antihyperlipidemic and antioxidant effects of ethanol and aqueous extracts of Cyclocarya paliurus leaves in type 2 diabetic rats. J. Ethnopharmacol. 2013, 150, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Yao, N.; Wang, Q.; Zhang, J.; Sun, Y.; Xiao, N.; Liu, K.; Huang, F.; Fang, S.; Shang, X.; et al. Cyclocarya paliurus extract modulates adipokine expression and improves insulin sensitivity by inhibition of inflammation in mice. J. Ethnopharmacol. 2014, 153, 344–351. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.F.; Meng, F.C.; Cao, L.J.; Jiang, C.H.; Zhao, M.G.; Shang, X.L.; Fang, S.Z.; Ye, W.C.; Zhang, Q.W.; Zhang, J.; et al. Triterpenoids from Cyclocarya paliurus and their inhibitory effect on the secretion of apoliprotein B48 in Caco-2 cells. Phytochemistry 2017, 142, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.M.; Yin, Z.Q.; Zhao, M.G.; Jiang, C.H.; Zhang, J.; Pan, K. Pentacyclic triterpenoids from Cyclocarya paliurus and their antioxidant activities in FFA-induced HepG2 steatosis cells. Phytochemistry 2018, 151, 119–127. [Google Scholar] [CrossRef]
- Zhu, K.N.; Jiang, C.H.; Tian, Y.S.; Xiao, N.; Wu, Z.F.; Ma, Y.L.; Lin, Z.; Fang, S.Z.; Shang, X.L.; Liu, K.; et al. Two triterpeniods from Cyclocarya paliurus (Batal) Iljinsk (Juglandaceae) promote glucose uptake in 3T3-L1 adipocytes: The relationship to AMPK activation. Phytomedicine 2015, 22, 837–846. [Google Scholar] [CrossRef]
- Cui, B.S.; Li, S. New triterpenoid saponins from the leaves of Cyclocarya paliurus. Chin. Chem. Lett. 2015, 26, 585–589. [Google Scholar] [CrossRef]
- Wang, Y.R.; Cui, B.S.; Han, S.W.; Li, S. New dammarane triterpenoid saponins from the leaves of Cyclocarya paliurus. J. Asian Nat. Prod. Res. 2018. [Google Scholar] [CrossRef]
- Ju, J.H.; Liu, D.; Lin, G.; Xu, X.D.; Han, B.; Yang, J.S.; Tu, G.Z.; Ma, L.B. Beesiosides A-F, six new cycloartane triterpene glycosides from Beesia calthaefolia. J. Nat. Prod. 2002, 65, 42–47. [Google Scholar] [CrossRef]
- Chen, Y.J.; Na, L.; Fan, J.; Zhao, J.; Hussain, N.; Jian, Y.Q.; Yuan, H.; Li, B.; Liu, B.; Choudhary, M.I.; et al. Seco-dammarane triterpenoids from the leaves of Cyclocarya paliurus. Phytochemistry 2018, 145, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Omae, A.; Okuyama, T. A new triterpenoid isolated from Lagerstronemia speciosa (L.) PERS. Chem. Pharm. Bull. 2003, 51, 452–454. [Google Scholar] [CrossRef] [PubMed]
- Jayasinghe, L.; Percy Wannigama, G.; Macleod, J.K. Triterpenoids from Anamirta cocculus. Phytochemistry 1993, 34, 1111–1116. [Google Scholar]
- Cui, B.S.; Li, S. Chemical constituents from leaves of Cyclocarya paliurus. Zhongcaoyao 2012, 43, 2132–2136. [Google Scholar]
- Chen, J.; Chen, B.; Tian, J.; Wu, F.E. Two new pentacyclic triterpenes from Sabia parviflora. Chin. Chem. Lett. 2002, 13, 345–348. [Google Scholar]
- Jiang, H.Z.; Ma, Q.Y.; Fan, H.J.; Liang, W.J.; Huang, S.Z.; Dai, H.F.; Wang, P.C.; Ma, X.F.; Zhao, Y.X. Fatty acid synthase inhibitors isolated from Punica granatum L. J. Braz. Chem. Soc. 2012, 23, 889–893. [Google Scholar] [CrossRef]
- Murakami, T.; Nagasawa, M.; Itokawa, H.; Tachi, Y.; Tanaka, K. The Structure of a new triterpene, momordic acid, obtained from Momordica cochinchinensis. Tetrahedron Lett. 1966, 42, 5137–5140. [Google Scholar] [CrossRef]
- Sang, S.; Lapsley, K.; Rosen, R.T.; Ho, C.T. New prenylated benzoic acid and other constituents from almond hulls (Prunus amygdalus Batsch). J. Agric. Food Chem. 2002, 50, 607–609. [Google Scholar] [CrossRef]
- Aguirre, M.C.; Delporte, C.; Backhouse, N.; Erazo, S.; Letelier, M.E.; Cassels, B.K.; Silva, X.; Alegría, S.; Negrete, R. Topical anti-inflammatory activity of 2α-hydroxy pentacyclic triterpene acids from the leaves of Ugnimolinae. Bioorg. Med. Chem. 2006, 14, 5673–5677. [Google Scholar] [CrossRef]
- Ren, S.G.; Xu, C.R.; Li, L.N. Studies on the sweet principles from the leaves of Cyclocarya paliurus. Acta Pharm. Sin. 1995, 30, 757–761. [Google Scholar]
- Jiang, Z.Y.; Zhang, X.M.; Zhou, J.; Qiu, S.X.; Chen, J.J. Two new triterpenoid glycosides from Cyclocarya paliurus. J. Asian Nat. Prod. Res. 2006, 8, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Miura, T.; Takagi, S.; Ishida, T. Management of diabetes and its complications with banaba (Lagerstroemia speciosa L.) and corosolic acid. J. Evid.-Based Complement. Altern. Med. 2012, 871495. [Google Scholar]
- Shi, L.; Zhang, W.; Zhou, Y.Y.; Zhang, Y.N.; Li, J.Y.; Hu, L.H.; Li, J. Corosolic acid stimulates glucose uptake via enhancing insulin receptor phosphorylation. Eur. J. Pharmacol. 2008, 584, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.H.; Kuo, Y.H.; Shih, C.C. Eburicoicacid, a triterpenoid compound from Antrodia camphorata, displays antidiabetic and antihyperlipidemic effects in palmitate-treated C2C12 myotubes and in high-fat diet-fed mice. Int. J. Mol. Sci. 2017, 18, 2314. [Google Scholar] [CrossRef] [PubMed]
- Towler, M.C.; Hardie, D.G. AMP-activated protein kinase in metabolic control andinsulin signaling. Circ. Res. 2007, 100, 328–341. [Google Scholar] [CrossRef] [PubMed]
- Zachariah Tom, R.; Garcia-Roves, P.M.; Sjögren, R.J.O.; Jiang, L.Q.; Holmström, M.H.; Deshmukh, A.S.; Vieira, E.; Chibalin, A.V.; Björnholm, M.; Zierath, J.R. Effects of AMPK activation on insulin sensitivity and metabolism in leptin-deficient ob/ob mice. Diabetes 2014, 63, 1560–1571. [Google Scholar] [CrossRef] [PubMed]
- Xi, X.; Han, J.; Zhang, J.Z. Stimulation of glucose transport by AMP-activated protein kinase via activation of p38 mitogen-activated protein kinase. J. Biol. Chem. 2001, 276, 41029–41034. [Google Scholar] [CrossRef]
- Somwar, R.; Koterski, S.; Sweeney, G.; Sciotti, R.; Djuric, S.; Berg, C.; Trevillyan, J.; Scherer, P.E.; Rondinone, C.M.; Klip, A. A dominant-negative p38 MAPK mutant and novel selective inhibitors of p38 MAPK reduce insulin-stimulated glucose uptake in 3T3-L1 adipocytes without affecting GLUT4 translocation. J. Biol. Chem. 2002, 277, 50386–50395. [Google Scholar] [CrossRef]
- Kim, S.H.; Hwang, J.T.; Park, H.S.; Kwon, D.Y.; Kim, M.S. Capsaicin stimulates glucose uptake in C2C12 muscle cells via the reactive oxygen species (ROS)/AMPK/p38 MAPK pathway. Biochem. Biophys. Res. Commun. 2013, 439, 66–70. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, J.M.; Kim, E.K.; Lee, J.O.; Lee, S.K.; Jung, J.H.; You, G.Y.; Park, S.H.; Suh, P.G.; Kim, H.S. Curcumin stimulates glucose uptake through AMPK-p38 MAPK pathways in L6 myotube cells. J. Cell.Physiol. 2010, 223, 771–778. [Google Scholar] [CrossRef]
- Cheng, Z.; Pang, T.; Gu, M.; Gao, A.H.; Xie, C.M.; Li, J.Y.; Nan, F.J.; Li, J. Berberine-stimulated glucose uptake in L6 myotubes involves both AMPK and p38 MAPK. Biochim. Biophys. Acta 2006, 1760, 1682–1689. [Google Scholar] [CrossRef] [PubMed]
- Yin, M.C.; Lin, M.C.; Mong, M.C.; Lin, C.Y. Bioavailability, distribution, and antioxidative effects of selected triterpenes in mice. J. Agric. Food Chem. 2012, 60, 7697–7701. [Google Scholar] [CrossRef] [PubMed]
- Cerga, O.; Borcan, F.; Ambrus, R.; Popovici, I. Syntheses of new cyclodextrin complexes with oleanolic and ursolic acids. J. Agroaliment. Process. Technol. 2011, 17, 405–409. [Google Scholar]
- Zhou, M.; Zhang, R.H.; Wang, M.; Xu, G.B.; Liao, S.G. Prodrugs of triterpenoids and their derivatives. Eur. J. Med. Chem. 2017, 131, 222–236. [Google Scholar] [CrossRef] [PubMed]
- Rautio, J.; Kumpulainen, H.; Heimbach, T.; Oliyai, R.; Oh, D.; Jarvinen, T.; Savolainen, J. Prodrugs: Design and clinical applications. Nat. Rev. Drug Discov. 2008, 7, 255–270. [Google Scholar] [CrossRef] [PubMed]
- Deepagan, V.G.; Thambi, T.; Ko, H.; Kang, Y.M.; Park, J.H. Amphiphilic polysialic acid derivatives: Synthesis, characterization, and in-vitro cytotoxicity. J. Nanosci. Nanotechnol. 2013, 13, 7312–7318. [Google Scholar] [CrossRef]
- Thambi, T.; Deepagan, V.G.; Ko, H.; Lee, D.S.; Park, J.H. Bioreducible polymersomes for intracellular dual-drug delivery. J. Mater. Chem. 2012, 22, 22028–22036. [Google Scholar] [CrossRef]
- Gan, L.S.; Zheng, D.J.; Liu, Q.; Zhou, J.; Zhang, M.Z.; Yao, W.; Shao, B.H.; Mo, J.X.; Zhou, C.X. Eight new cycloartane triterpenoids from Beesia calthifolia with hepatoprotective effects against D-galactosamine induced L02 cell damage. Bioorg. Med. Chem. Lett. 2015, 25, 3845–3849. [Google Scholar] [CrossRef]
- Liu, J.X.; Shen, S.N.; Tong, Q.; Wang, Y.T.; Lin, L.G. Honokiol protects hepatocytes from oxidative injury through mitochondrial deacetylase SIRT3. Eur. J. Pharmacol. 2018, 834, 176–187. [Google Scholar] [CrossRef]
- Shen, S.N.; Liao, Q.W.; Feng, Y.; Liu, J.X.; Pan, R.L.; Lee, S.M.Y.; Lin, L.G. Myricanol mitigates lipid accumulation in 3T3-L1 adipocytes and high fat diet-fed zebrafish via activating AMP-activated protein kinase. Food Chem. 2019, 270, 305–314. [Google Scholar] [CrossRef]
- Li, D.; Liu, Q.Y.; Sun, W.; Chen, X.P.; Wang, Y.; Sun, Y.X.; Lin, L.G. 1,3,6,7-tetrahydroxy-8-prenylxanthone ameliorates inflammatory responses resulting from the paracrine interaction of adipocytes and macrophages. Br. J. Pharmacol. 2018, 175, 1590–1606. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of all the isolated compounds are available from the authors. |






| No. | 1 | 2 | 3 | 4 | ||||
|---|---|---|---|---|---|---|---|---|
| δH | δC | δH | δC | δH | δC | δH | δC | |
| 1 | a 1.53, m | 38.4 | a 1.47, m | 38.3 | a 1.42, m | 38.0 | 215.4 | |
| b 2.52, m | b 2.53, dt (13.3, 5.0) | b 2.46, m | ||||||
| 2 | a 2.26, m | 30.8 | a 2.29, dt (13.3, 3.5) | 30.7 | a 2.27, dt (13.3, 3.3) | 30.5 | α 2.29, dd (11.9, 4.9) | 44.8 |
| b 2.66, dt (12.9, 5.2) | b 2.74, dt (13.0, 5.0) | b 2.74, dt (13.3, 5.1) | β 3.12, t (11.9) | |||||
| 3 | 180.2 | 178.3 | 177.9 | 3.82, dd (12.1, 4.9) | 73.5 | |||
| 4 | 149.3 | 149.3 | 149.3 | 44.1 | ||||
| 5 | 2.05, dd (12.7, 2.8) | 53.0 | 2.04, dd (12.8, 2.9) | 53.1 | 2.04, dd (12.8, 2.9) | 53.1 | 1.39, m | 47.9 |
| 6 | α 1.86, m | 27.4 | α 1.85, m | 27.4 | α 1.85, m | 27.5 | α 1.57, m | 18.6 |
| β 1.45, m | β 1.49, m | β 1.47, m | β 1.57, m | |||||
| 7 | α 1.59, m | 35.8 | α 1.59, dt (13.0, 3.3) | 35.8 | α 1.59, dt (12.9, 3.3) | 35.7 | α 1.55, m | 33.3 |
| β 1.22, m | β 1.21, m | β 1.22, m | β 1.29, m | |||||
| 8 | 40.9 | 40.9 | 40.9 | 40.5 | ||||
| 9 | 1.95, m | 45.4 | 1.95, m | 45.4 | 1.93, m | 45.5 | 2.40, m | 40.8 |
| 10 | 41.9 | 41.9 | 41.9 | 53.6 | ||||
| 11 | α 2.46, m | 33.8 | α 2.46, dt (12.5, 4.1) | 34.0 | α 2.42, m | 34.3 | α 2.44, m | 26.5 |
| β 1.34, m | β 1.35, m | β 1.37, m | β 1.88, ddd (12.5, 11.3, 5.4) | |||||
| 12 | 4.07, dt (10.7, 4.7) | 76.5 | 4.05, dt (10.8, 4.9) | 76.6 | 4.03, dt (10.8, 5.0) | 76.5 | 5.30, m | 125.3 |
| 13 | 1.70, m | 41.9 | 1.70, m | 41.9 | 1.70, m | 41.8 | 144.0 | |
| 14 | 51.4 | 51.4 | 51.4 | 42.8 | ||||
| 15 | α 1.49, m | 32.3 | α 1.44, m | 32.3 | α 1.45, m | 32.4 | α 1.00, m | 29.6 |
| β 1.13, m | β 1.12, m | β 1.12, m | β 1.72, m | |||||
| 16 | α 1.99, m | 27.1 | α 1.98, m | 27.1 | α 1.98, m | 27.0 | α 2.25, m | 28.7 |
| β 1.81, m | β 1.80, m | β 1.80, m | β 1.59. m | |||||
| 17 | 1.92, m | 50.2 | 1.93, m | 50.2 | 1.92, m | 50.1 | 47.0 | |
| 18 | 1.06, s | 16.9 | 1.05, s | 16.9 | 1.05, s | 16.8 | 3.06, br s | 45.5 |
| 19 | 1.10, s | 20.6 | 1.09, s | 20.6 | 1.09, s | 20.5 | 3.27, d (3.6) | 82.4 |
| 20 | 87.8 | 87.8 | 87.9 | 36.1 | ||||
| 21 | 1.16, s | 25.5 | 1.16, s | 25.5 | 1.16, s | 25.2 | α 1.64, m | 29.6 |
| β 1.00, m | ||||||||
| 22 | α 1.73, m | 35.0 | α 1.74, m | 35.0 | α 1.73, m | 34.8 | α 1.62, m | 34.0 |
| β 1.65, m | β 1.67, m | β 1.66, m | β 1.78, m | |||||
| 23 | α 1.89, m | 26.1 | α 1.89, m | 26.1 | α 1.89, m | 26.1 | a 3.33, d (11.2) | 65.9 |
| β 1.31, m | β 1.31, m | β 1.32, m | b 3.50, d (11.2) | |||||
| 24 | 3.79, m | 84.9 | 3.79, m | 84.9 | 3.79, dd (7.8, 6.6) | 84.9 | 0.88, s | 13.2 |
| 25 | 72.7 | 72.6 | 72.8 | 1.35, s | 15.9 | |||
| 26 | 1.19, s | 26.6 | 1.19, s | 26.6 | 1.21, s | 26.8 | 0.85, s | 18.2 |
| 27 | 1.16, s | 24.4 | 1.16, s | 24.5 | 1.15, s | 24.6 | 1.33, s | 25.1 |
| 28 | a 4.72, d (1.4) | 114.1 | a 4.71, d (1.6) | 114.1 | a 4.70, d (1.6) | 114.0 | 182.9 | |
| b 4.85, d (1.4) | b 4.84, d (1.6) | b 4.84, d (1.6) | ||||||
| 29 | 1.77, s | 24.0 | 1.76, s | 24.0 | 1.76, s | 23.9 | 0.94, s | 28.7 |
| 30 | 1.00, s | 16.9 | 1.00, s | 16.9 | 0.99, s | 16.9 | 0.97, s | 25.2 |
| 1′ | 4.29, d (7.1) | 101.2 | 4.28, d (7.1) | 101.4 | 4.36, d (7.7) | 100.4 | ||
| 2′ | 3.50, dd (9.6, 7.1) | 72.8 | 3.50, dd (9.6, 7.1) | 72.8 | 3.18, dd (9.3, 7.7) | 75.3 | ||
| 3′ | 3.46, dd (9.6, 3.3) | 74.6 | 3.46, dd (9.6, 3.3) | 74.6 | 3.29, m | 77.8 | ||
| 4′ | 3.77, m | 70.4 | 3.77, m | 70.3 | 3.00, t (9.1) | 77.1 | ||
| 5′ | 3.54, dd (12.7, 0.8) | 67.8 | 3.54, dd (12.7, 1.1) | 67.7 | 3.26, m | 73.0 | ||
| 3.88, dd (12.7, 1.9) | 3.88, dd (12.7, 1.9) | |||||||
| 6′ | 1.26, d (6.1) | 18.1 | ||||||
| OCH3 | 3.64, s | 52.1 | 3.63, s | 52.0 | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, Z.-J.; Shen, S.-N.; Wang, J.-M.; Wu, Y.-J.; Zhou, C.-X.; Mo, J.-X.; Lin, L.-G.; Gan, L.-S. Triterpenoids from Cyclocarya paliurus that Enhance Glucose Uptake in 3T3-L1 Adipocytes. Molecules 2019, 24, 187. https://doi.org/10.3390/molecules24010187
Fang Z-J, Shen S-N, Wang J-M, Wu Y-J, Zhou C-X, Mo J-X, Lin L-G, Gan L-S. Triterpenoids from Cyclocarya paliurus that Enhance Glucose Uptake in 3T3-L1 Adipocytes. Molecules. 2019; 24(1):187. https://doi.org/10.3390/molecules24010187
Chicago/Turabian StyleFang, Zhu-Jun, Sheng-Nan Shen, Jia-Min Wang, Yong-Jiang Wu, Chang-Xin Zhou, Jian-Xia Mo, Li-Gen Lin, and Li-She Gan. 2019. "Triterpenoids from Cyclocarya paliurus that Enhance Glucose Uptake in 3T3-L1 Adipocytes" Molecules 24, no. 1: 187. https://doi.org/10.3390/molecules24010187
APA StyleFang, Z. -J., Shen, S. -N., Wang, J. -M., Wu, Y. -J., Zhou, C. -X., Mo, J. -X., Lin, L. -G., & Gan, L. -S. (2019). Triterpenoids from Cyclocarya paliurus that Enhance Glucose Uptake in 3T3-L1 Adipocytes. Molecules, 24(1), 187. https://doi.org/10.3390/molecules24010187