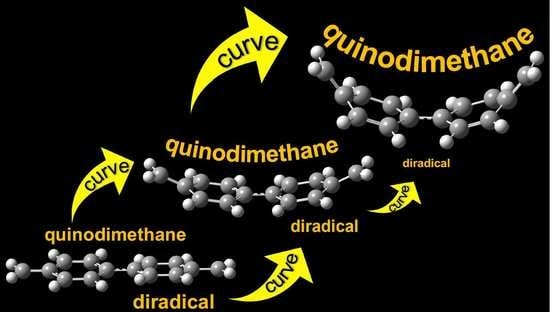
Curve Effect on Singlet Diradical Contribution in Kekulé-type Diradicals: A Sensitive Probe for Quinoidal Structure in Curved π-Conjugated Molecules
Abstract
:1. Introduction
2. Results and Discussion
2.1. Computations for DR1
2.2. Computations for DR2-4
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Barth, W.E.; Lawton, R.G. Dibenzo[ghi,mno]fluoranthene. J. Am. Chem. Soc. 1966, 88, 380–381. [Google Scholar] [CrossRef]
- Kroto, H.W.; Heath, J.R.; Obrien, S.C.; Curl, R.F.; Smalley, R.E. C-60—Buckminsterfullerene. Nature 1985, 318, 162–163. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Scott, L.T. Fragments of fullerenes: Novel syntheses, structures and reactions. Pure Appl. Chem. 1996, 68, 291–300. [Google Scholar] [CrossRef] [Green Version]
- Sakurai, H.; Daiko, T.; Hirao, T. A synthesis of sumanene, a fullerene fragment. Science 2003, 301, 1878. [Google Scholar] [CrossRef] [PubMed]
- Scott, L.T. Methods for the Chemical Synthesis of Fullerenes. Angew. Chem. Int. Ed. 2004, 43, 4994–5007. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.T.; Siegel, J.S. Aromatic molecular-bowl hydrocarbons: Synthetic derivatives, their structures, and physical properties. Chem. Rev. 2006, 106, 4843–4867. [Google Scholar] [CrossRef]
- Akasaka, T.; Osuka, A.; Fukuzumi, S.; Kandori, H.; Aso, Y. (Eds.) Chemical Science of π-Electron Systems; Springer: Tokyo, Japan, 2015. [Google Scholar]
- Nestoros, E.; Stuparu, M.C. Corannulene: A molecular bowl of carbon with multifaceted properties and diverse applications. Chem. Commun. 2018, 54, 6503–6519. [Google Scholar] [CrossRef]
- Márquez, I.R.; Castro-Ferández, S.; Millán, A.; Campaña, A.G. Synthesis of distorted nanographenes containing seven- and eight-membered carbocycles. Chem. Commun. 2018, 54, 6705–6718. [Google Scholar] [CrossRef]
- Sun, Z.; Matsuno, T.; Isobe, H. Stereoisomerism and Structures of Rigid Cylindrical Cycloarylenes. Bull. Chem. Soc. Jpn. 2018, 91, 907–921. [Google Scholar] [CrossRef]
- Meier, H.; Stalmach, U.; Kolshorn, H. Effective conjugation length and UV/vis spectra of oligomers. Acta Polym. 1997, 48, 379–384. [Google Scholar] [CrossRef]
- Kammermeier, S.; Jones, P.G.; Herges, R. Ring-Expanding Metathesis of Tetradehydro- anthracene-Synthesis and Structure of a Tubelike, Fully Conjugated Hydrocarbon. Angew. Chem. Int. Ed. 1996, 35, 2669–2671. [Google Scholar] [CrossRef]
- Kawase, T.; Kurata, H. Ball-, bowl-, and belt-shaped conjugated systems and their complexing abilities: Exploration of the concave-convex π-π interaction. Chem. Rev. 2006, 106, 5250–5273. [Google Scholar] [CrossRef] [PubMed]
- Tahara, K.; Tobe, Y. Molecular loops and belts. Chem. Rev. 2006, 106, 5274–5290. [Google Scholar] [CrossRef] [PubMed]
- Jasti, R.; Bhattacharjee, J.; Neaton, J.B.; Bertozzi, C.R. Synthesis, Characterization, and Theory of [9]-, [12]-, and [18]Cycloparaphenylene: Carbon Nanohoop Structures. J. Am. Chem. Soc. 2008, 130, 17646–17647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takaba, H.; Omachi, H.; Yamamoto, Y.; Bouffard, J.; Itami, K. Selective Synthesis of 12 Cycloparaphenylene. Angew. Chem. Int. Ed. 2009, 48, 6112–6116. [Google Scholar] [CrossRef] [PubMed]
- Yamago, S.; Watanabe, Y.; Iwamoto, T. Synthesis of 8 Cycloparaphenylene from a Square-Shaped Tetranuclear Platinum Complex. Angew. Chem. Int. Ed. 2010, 49, 757–759. [Google Scholar] [CrossRef] [PubMed]
- Omachi, H.; Matsuura, S.; Segawa, Y.; Itami, K. A Modular and Size-Selective Synthesis of n Cycloparaphenylenes: A Step toward the Selective Synthesis of n, n Single-Walled Carbon Nanotubes. Angew. Chem. Int. Ed. 2010, 49, 10202–10205. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, T.; Watanabe, Y.; Sakamoto, Y.; Suzuki, T.; Yamago, S. Selective and Random Syntheses of n Cycloparaphenylenes (n = 8–13) and Size Dependence of Their Electronic Properties. J. Am. Chem. Soc. 2011, 133, 8354–8361. [Google Scholar] [CrossRef] [PubMed]
- Segawa, Y.; Fukazawa, A.; Matsuura, S.; Omachi, H.; Yamaguchi, S.; Irle, S.; Itami, K. Combined experimental and theoretical studies on the photophysical properties of cycloparaphenylenes. Org. Biomol. Chem. 2012, 10, 5979–5984. [Google Scholar] [CrossRef]
- Wong, B.M. Optoelectronic Properties of Carbon Nanorings: Excitonic Effects from Time-Dependent Density Functional Theory. J. Phys. Chem. C 2009, 113, 21921–21927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golder, M.R.; Jasti, R. Syntheses of the Smallest Carbon Nanohoops and the Emergence of Unique Physical Phenomena. Acc. Chem. Res. 2015, 48, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Darzi, E.R.; Jasti, R. The dynamic, size-dependent properties of 5–12 cycloparaphenylenes. Chem. Soc. Rev. 2015, 44, 6401–6410. [Google Scholar] [CrossRef]
- Segawa, Y.; Yagi, A.; Matsui, K.; Itami, K. Design and Synthesis of Carbon Nanotube Segments. Angew. Chem. Int. Ed. 2016, 55, 5136–5158. [Google Scholar] [CrossRef] [PubMed]
- Fujitsuka, M.; Iwamoto, T.; Kayahara, E.; Yamago, S.; Majima, T. Enhancement of the Quinoidal Character for Smaller n Cycloparaphenylenes Probed by Raman Spectroscopy. ChemPhysChem 2013, 14, 1570–1572. [Google Scholar] [CrossRef] [PubMed]
- Fujitsuka, M.; Cho, D.W.; Iwamoto, T.; Yamago, S.; Majima, T. Size-dependent fluorescence properties of n cycloparaphenylenes (n = 8–13), hoop-shaped pi-conjugated molecules. Phys. Chem. Chem. Phys. 2012, 14, 14585–14588. [Google Scholar] [CrossRef]
- Fujitsuka, M.; Lu, C.; Iwamoto, T.; Kayahara, E.; Yamago, S.; Majima, T. Properties of Triplet-Excited n Cycloparaphenylenes (n = 8–12): Excitation Energies Lower than Those of Linear Oligomers and Polymers. J. Phys. Chem. A 2014, 118, 4527–4532. [Google Scholar] [CrossRef]
- Jenneskens, L.W.; Vaneenige, E.N.; Louwen, J.N. A P-orbital axis vector (POAV) analysis of boat-shaped benzenes. New J. Chem. 1992, 16, 775–779. [Google Scholar]
- Tobe, Y. Strained N Cyclophanes. Cyclophanes 1994, 172, 1–40. [Google Scholar]
- Bickelhaupt, F. Small cyclophanes—The bent benzene business. Pure Appl. Chem. 1990, 62, 373–382. [Google Scholar] [CrossRef]
- Dewar, M.J.S.; Wade, L.E. Study of mechanism of cope rearrangement. J. Am. Chem. Soc. 1977, 99, 4417–4424. [Google Scholar] [CrossRef]
- Thiele, J.; Balhorn, H. Ueber einen chinoïden Kohlenwasserstoff. Chem. Berichte 1904, 37, 1463–1470. [Google Scholar] [CrossRef]
- Tschichibabin, A.E. Über einige phenylierte derivate des p,p-Ditolyls. Chem. Berichte 1907, 40, 1810–1819. [Google Scholar] [CrossRef]
- Müller, E.; Pfanz, H. Über biradikaloide Terphenylderivate. Chem. Berichte 1941, 74, 1051–1074. [Google Scholar] [CrossRef]
- Müller, E.; Hermann, P. Über ein biradikaloides Quterphenylderivat. Chem. Berichte 1941, 74, 1075–1083. [Google Scholar] [CrossRef]
- Schmidt, R.; Brauer, H.-D. The Energetic Positions of the Lowest Singlet and Triplet State of the Schlenk and of the Müller Hydrocarbon. Angew. Chem. Int. Ed. Engl. 1971, 10, 506. [Google Scholar] [CrossRef]
- Doering, W.V.E.; Toscano, V.G.; Beasley, G.H. Kinetics of cope rearrangement of 1,1-dideuteriohexa-1,5-diene. Tetrahedron 1971, 27, 5299–5306. [Google Scholar] [CrossRef]
- Kolc, J.; Michl, J. Pi,Pi-Biradicaloid Hydrocarbons—Pleiadene Family. I. Photochemical Preparation from Cyclobutene Precursors. J. Am. Chem. Soc. 1973, 95, 7391–7401. [Google Scholar] [CrossRef]
- Michl, J.; Bonacickoutecky, V. Biradicals and biradicaloids—A unified view. Tetrahedron 1988, 44, 7559–7585. [Google Scholar] [CrossRef]
- Shimizu, A.; Tobe, Y. Indeno 2,1-a fluorene: An Air-Stable ortho-Quinodimethane Derivative. Angew. Chem. Int. Ed. 2011, 50, 6906–6910. [Google Scholar] [CrossRef]
- Abe, M. Diradicals. Chem. Rev. 2013, 113, 7011–7088. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Zeng, Z.B.; Wu, J.S. Zethrenes, Extended p-Quinodimethanes, and Periacenes with a Singlet Biradical Ground State. Acc. Chem. Res. 2014, 47, 2582–2591. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Z.B.; Shi, X.L.; Chi, C.Y.; Navarrete, J.T.L.; Casado, J.; Wu, J.S. Pro-aromatic and anti-aromatic π-conjugated molecules: An irresistible wish to be diradicals. Chem. Soc. Rev. 2015, 44, 6578–6596. [Google Scholar] [CrossRef] [PubMed]
- Kubo, T. Recent Progress in Quinoidal Singlet Biradical Molecules. Chem. Lett. 2015, 44, 111–122. [Google Scholar] [CrossRef] [Green Version]
- Frederickson, C.K.; Zalcharov, L.N.; Haley, M.M. Modulating Paratropicity Strength in Diareno-Fused Antiaromatics. J. Am. Chem. Soc. 2016, 138, 16827–16838. [Google Scholar] [CrossRef] [PubMed]
- Konishi, A.; Okada, Y.; Nakano, M.; Sugisaki, K.; Sato, K.; Takui, T.; Yasuda, M. Synthesis and Characterization of Dibenzo[a,f]pentalene: Harmonization of the Antiaromatic and Singlet Biradical Character. J. Am. Chem. Soc. 2017, 139, 15284–15287. [Google Scholar] [CrossRef] [PubMed]
- Frederickson, C.K.; Rose, B.D.; Haley, M.M. Explorations of the Indenofluorenes and Expanded Quinoidal Analogues. Acc. Chem. Res. 2017, 50, 977–987. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Yuan, N.N.; Fang, Y.; Chen, C.; Wang, L.; Feng, R.; Zhao, Y.; Cui, H.Y.; Wang, X.P. Studies on the Bridge Dependence of Bis(triarylamine) Diradical Dications: Long-Range π-Conjugation and π-π Coupling Systems. J. Org. Chem. 2018, 83, 3651–3656. [Google Scholar] [CrossRef]
- Li, G.; Gopalakrishna, T.Y.; Phan, H.; Herng, T.S.; Ding, J.; Wu, J. From Open-shell Singlet Diradicaloid to Closed-Shell Global Antiaromatic Macrocycles. Angew. Chem. Int. Ed. 2018, 57, 7166–7170. [Google Scholar] [CrossRef]
- Dressler, J.J.; Teraoka, M.; Espejo, G.L.; Kishi, R.; Takamuku, S.; Gómez-Garcia, C.J.; Zakharov, L.N.; Nakano, M.; Casado, J.; Haley, M.M. Thiophene and its sulfur inhibit indenoindenodibenzothiophene diradicals from low-energy lying thermal triplets. Nat. Chem. 2018, 10, 1134–1140. [Google Scholar] [CrossRef]
- Ravat, P.; Solomek, T.; Haussinger, D.; Blacque, O.; Juricek, M. Dimethylcethrene: A Chiroptical Diradicaloid Photoswitch. J. Am. Chem. Soc. 2018, 140, 10839–10847. [Google Scholar] [CrossRef] [PubMed]
- Tokunaga, A.; Mutoh, K.; Hasegawa, T.; Abe, J. Reversible Valence Photoisomerization between Closed-Shell Quinoidal and Open-Shell Biradical Forms. J. Phys. Chem. Lett. 2018, 9, 1833–1837. [Google Scholar] [CrossRef] [PubMed]
- Rottschafer, D.; Ho, N.K.T.; Neumann, B.; Stammler, H.G.; van Gastel, M.; Andrada, D.M.; Ghadwal, R.S. N-Heterocyclic Carbene Analogues of Thiele and Chichibabin Hydrocarbons. Angew. Chem. Int. Ed. 2018, 57, 5838–5842. [Google Scholar] [CrossRef] [PubMed]
- Hansmann, M.M.; Melaimi, M.; Munz, D.; Bertrand, G.J. Modular Approach to Kekulé Diradicaloids Derived from Cyclic (Alkyl)(amino)carbenes. Am. Chem. Soc. 2018, 140, 2546–2554. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, K.; Jensen, F.; Dorigo, A.; Houk, K.N. A spin correction procedure for unrestricted Hartree-Fock and Møller-Plesset wavefunctions for singlet diradicals and polyradicals. Chem. Phys. Lett. 1988, 149, 537–542. [Google Scholar] [CrossRef]
- Hegarty, D.; Robb, M.A. Application of unitary group-methods to configuration-interaction calculations. Mol. Phys. 1979, 38, 1795–1812. [Google Scholar] [CrossRef]
- Woon, D.E.; Dunning, T.H. Gaussian-basis sets for use in correlated molecular calculations. 3. the atoms aluminum through argon. J. Chem. Phys. 1993, 98, 1358–1371. [Google Scholar] [CrossRef]
- Wiberg, K.B. Application of the Pople-Santry-Segal CNDO Method to the Cyclopropylcarbinyl and Cyclobutyl Cation and to Bicyclobutane. Tetrahedron 1966, 24, 1083–1096. [Google Scholar] [CrossRef]
- Weinhold, F.; Landis, C.R. Discovering Chemistry with Natural Bond Orbitals; Wiley: New York, NY, USA, 2012. [Google Scholar]
- Andersson, K.; Malmqvist, P.A.; Roos, B.O.; Sadlej, A.J.; Wolinski, K. 2nd-order perturbation-theory with a CASSCF reference function. J. Phys. Chem. 1990, 94, 5483–5488. [Google Scholar] [CrossRef]
- Andersson, K.; Malmqvist, P.A.; Roos, B.O. 2nd-order perturbation-theory with a complete active space self-consistent field reference function. J. Chem. Phys. 1992, 96, 1218–1226. [Google Scholar] [CrossRef]



| Entry | DR | Bent Angle θ (°) | Occupation Number | q | BO b | C1–C2 Singlet/Triplet | ΔEST c ΔErel,S/ΔErel,T | |
|---|---|---|---|---|---|---|---|---|
| ψA (HOMO) | ψB (LUMO) | |||||||
| 1 | DR1 | 0 (C1-C2-C6 = C10-C9-C5 = 180°) | 1.66 | 0.35 | 83.0 | 1.55 | 137.4/140.8 | 11.6 0.0/0.0 |
| 2 | 13 (160°) | 1.70 | 0.31 | 85.0 | 1.61 | 136.7/140.8 | 12.9 +4.4/+5.7 | |
| 3 | 25 (140°) | 1.79 | 0.23 | 89.5 | 1.73 | 135.6/140.4 | 17.4 +17.0/+22.8 | |
| 4 | 29 (135°) | 1.80 | 0.21 | 90.0 | 1.75 | 135.5/140.2 | 19.3 +21.0/+28.7 | |
| 5 | DR2 | 0 (C1-C2-C6 = C8-C7-C12 = 180°) | 1.77 | 0.24 | 88.5 | 1.68 | 135.9/142.0 | 22.0 0.0/0.0 |
| 6 | 12 (160°) | 1.78 | 0.23 | 89.0 | 1.69 | 135.8/142.0 | 23.3 +4.0/+5.2 | |
| 7 | 17 (140°) | 1.82 | 0.19 | 91.0 | 1.71 | 135.6/141.9 | 27.4 +15.4/+20.7 | |
| 8 | 26 (120°) | 1.85 | 0.16 | 92.5 | 1.76 | 135.2/141.6 | 35.5 +32.4/+45.8 | |
| 9 | DR3 | 0 (C1-C5-C6 = C10-C6-C5 = 180°) | 1.58 | 0.43 | 79.0 | 1.47 | 138.5/141.2 | 11.8 +0.0/+0.0 |
| 10 | 17 (160°) | 1.62 | 0.39 | 81.0 | 1.51 | 138.1/141.1 | 12.9 +5.9/+7.0 | |
| 11 | 34 (140°) | 1.76 | 0.25 | 88.0 | 1.67 | 136.2/140.8 | 16.6 +23.1/+27.9 | |
| 12 | 52 (120 °) | 1.87 | 0.14 | 93.5 | 1.75 | 135.6/140.2 | 26.7 +46.6/+61.4 | |
| 13 | DR4 | 0 (C1-C5-C6 = C10-C13-C14 = 180°) | 1.65 | 0.36 | 82.5 | 1.54 | 137.5/140.6 | 15.2 +0.0/+0.0 |
| 14 | 12 (160°) | 1.68 | 0.33 | 84.0 | 1.57 | 137.2/140.5 | 16.0 +5.0/+5.8 | |
| 15 | 24 (140°) | 1.76 | 0.25 | 88.0 | 1.68 | 136.0/140.3 | 18.3 +20.1/+23.2 | |
| 16 | 35 (120°) | 1.82 | 0.19 | 91.0 | 1.73 | 135.6/140.0 | 24.0 +43.0/+51.7 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsumoto, M.; Antol, I.; Abe, M. Curve Effect on Singlet Diradical Contribution in Kekulé-type Diradicals: A Sensitive Probe for Quinoidal Structure in Curved π-Conjugated Molecules. Molecules 2019, 24, 209. https://doi.org/10.3390/molecules24010209
Matsumoto M, Antol I, Abe M. Curve Effect on Singlet Diradical Contribution in Kekulé-type Diradicals: A Sensitive Probe for Quinoidal Structure in Curved π-Conjugated Molecules. Molecules. 2019; 24(1):209. https://doi.org/10.3390/molecules24010209
Chicago/Turabian StyleMatsumoto, Misaki, Ivana Antol, and Manabu Abe. 2019. "Curve Effect on Singlet Diradical Contribution in Kekulé-type Diradicals: A Sensitive Probe for Quinoidal Structure in Curved π-Conjugated Molecules" Molecules 24, no. 1: 209. https://doi.org/10.3390/molecules24010209
APA StyleMatsumoto, M., Antol, I., & Abe, M. (2019). Curve Effect on Singlet Diradical Contribution in Kekulé-type Diradicals: A Sensitive Probe for Quinoidal Structure in Curved π-Conjugated Molecules. Molecules, 24(1), 209. https://doi.org/10.3390/molecules24010209