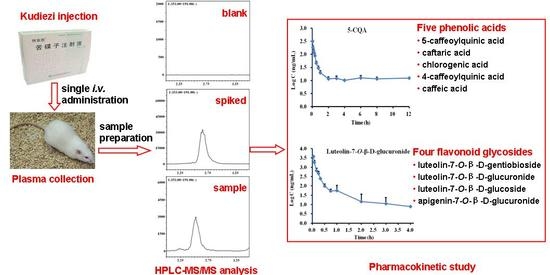
Simultaneous Determination of Five Phenolic Acids and Four Flavonoid Glycosides in Rat Plasma Using HPLC-MS/MS and Its Application to a Pharmacokinetic Study after a Single Intravenous Administration of Kudiezi Injection
Abstract
:1. Introduction
2. Results and Discussion
2.1. Method Validation
2.1.1. Selectivity
2.1.2. Calibration Curves and Linearity
2.1.3. Accuracy and Precision
2.1.4. Extraction Recoveries and Matrix Effects
2.1.5. Stability
2.1.6. Pharmacokinetic Study
2.2. Discussion
3. Materials and Methods
3.1. Materials and Reagents
3.2. Animals
3.3. HPLC-MS/MS Instrumentation and Conditions
3.4. Plasma Sample Preparation
3.5. Method Validation
3.5.1. Selectivity
3.5.2. Calibration Curves and Linearity
3.5.3. Accuracy and Precision
3.5.4. Extraction Recoveries and Matrix Effects
3.5.5. Stability
3.5.6. Pharmacokinetic Experiment
3.6. Data processing
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Han, F.; Li, Y.T.; Zhang, X.S.; Song, A.H.; Zhang, J.D.; Yin, R. Comparative study of direct injection analysis and liquid chromatography mass spectrometry for identification of chemical constituents in Kudiezi injection by FT-ICR MS. Int. J. Mass Spectrom. 2016, 405, 32–38. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, J.Y.; Yin, P.H.; Wang, Z.J.; Dong, L.Y.; Lu, J.Q. Rapid identification of polyphenols in Kudiezi injection with a practical technique of mass defect filter based on high-performance liquid chromatography coupled with linear ion trap/orbitrap mass spectrometry. Anal. Methods 2014, 6, 3515–3523. [Google Scholar] [CrossRef]
- Liu, R.R.; Zhang, X.P.; Wang, F.; Shang, Z.P.; Wang, F.; Liu, Y.; Lu, J.Q.; Zhang, J.Y. Rapid screening and identification of sesquiterpene lactones in Kudiezi injection based on high-performance liquid chromatography coupled with linear ion trap-orbitrap mass spectrometry. Chin. J. Nat. Med. 2018, 16, 150–160. [Google Scholar] [CrossRef]
- He, X.J.; Wang, C.X.; Cao, S.J.; Liu, Y.L. Protective effects of Kudiezi injection on acute myocardial ischemia. Acad. J. Guangdong Coll. Pharm. 2005, 21, 555–556, 562. [Google Scholar]
- Zhou, X.M.; Jin, W.S.; Cao, C.Y.; Cao, Y.L. The protective effect of Kudiezi injection on the experimental acute myocardial infarction. Lishizhen Med. Mater. Med. Res. 2007, 18, 122–123, 126. [Google Scholar]
- Liu, S.Y.; Cai, W.; Wang, F.; Liu, Y.; Shang, Z.P.; Zhang, X.P.; Wang, Z.J.; Lu, J.Q.; Zhang, J.Y. UHPLC-LTQ-Orbitrap-based metabolomics coupled with metabolomics pathway analysis method for exploring the protection mechanism of Kudiezi injection in a rat anti-ischemic cerebral reperfusion damage model. Chin. J. Nat. Med. 2017, 15, 955–960. [Google Scholar] [CrossRef]
- Liu, X.M.; Zhang, X.Y.; Wang, F.L.; Liang, X.; Zeng, Z.X.; Zhao, J.Y.; Zheng, H.; Jiang, X.N.; Zhang, Y.L. Improvement in cerebral ischemia-reperfusion injury through the TLR4/NF kappa B pathway after Kudiezi injection in rats. Life Sci. 2017, 191, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Y.; Yin, Y.Z.; Sui, D.Y.; Yu, X.F.; Qu, S.C.; Jiang, Y. The effect of DMLI on blood lipid metabolism in hyperlipidemia rats. J. Jilin Univ. (Med. Ed.) 2002, 28, 357–359. [Google Scholar]
- Gao, S.; Zhou, J. Advances in studies on clinical application of Kudiezi Injection. Drugs Clin. 2012, 27, 180–184. [Google Scholar]
- Liao, X.; Zeng, X.B.; Xie, Y.M.; Yang, W.; Yang, W. Use propensity score method to analyze data about effectiveness of coronary heart disease treated by Kudiezi injection. Zhongguo Zhong Yao Za Zhi 2013, 38, 3172–3179. [Google Scholar]
- He, Z.C.; Yan, H.M.; Li, G.C.; Li, J.F. Meta analysis on Kudiezi Injection in the treatment for angina pectoris. Chin. Tradit. Pat. Med. 2014, 36, 253–258. [Google Scholar]
- Yu, Z.A.; Yu, S.Y.; Hu, W.P.; Yu, F.; Yu, S.Y. Clinical observation on protective effect of Yueanxin Injection on the viable ischemic myocardium in patients with acute myocardial infarction. Chin. J. Geriatr. Heart Brain Vessel Dis. 2005, 7, 27–29. [Google Scholar]
- Liao, X.; Yu, D.D.; Xie, Y.M.; Zhang, Y.L.; He, Y.; Zhang, Y.; Liu, Y.; Yi, D.H.; Wang, Y.Y. Post-marketing safety surveillance of Diemailing Kudiezi injection: Real world study in 30 233 cases. Zhongguo Zhong Yao Za Zhi 2017, 42, 2857–2863. [Google Scholar] [PubMed]
- Liu, C.X. Difficulty and hot-points on pharmacokinetics studies of traditional Chinese medicine. Yao Xue Xue Bao 2005, 40, 395–401. [Google Scholar] [PubMed]
- Yin, R.; Chen, X.H.; Han, F.; Shen, Z.D.; Cheng, W.M.; Bi, K.S. LC-MS determination and pharmacokinetic study of luteolin-7-O-β-D-glucoside in rat plasma after administration of the traditional chinese medicinal preparation Kudiezi Injection. Chromatographia 2008, 67, 961–965. [Google Scholar] [CrossRef]
- Yin, R.; Han, F.; Tang, Z.; Liu, R.; Zhao, X.; Chen, X.H.; Bi, K.S. UFLC-MS/MS method for simultaneous determination of luteolin-7-O-gentiobioside, luteolin-7-O-β-d-glucoside and luteolin-7-O-β-d-glucuronide in beagle dog plasma and its application to a pharmacokinetic study after administration of traditional Chinese medicinal preparation: Kudiezi injection. J. Pharm. Biomed. Anal. 2013, 72, 127–133. [Google Scholar] [PubMed]
- Yin, L.; Shi, M.Y.; Sun, Y.T.; Sun, X.C.; Meng, H.; Fawcett, J.P.; Yang, Y.; Gu, J.K. A liquid chromatography-tandem mass spectrometric method for the simultaneous quantitation of five components of Ixeris sonchifoliain (Bge.) Hance in rat plasma and its application to a pharmacokinetic study. J. Chromatogr. B 2013, 931, 12–16. [Google Scholar] [CrossRef]
- Liu, Y.; Lu, J.Q.; Zhang, J.Y.; Wang, Q.; Wang, F.; Qiao, Y.J.; Zhang, Y.L. Rapid determination of ten polyphenols in Kudiezi injection using ultra-performance liquid chromatography-tandem mass spectrometry in multiple reaction monitoring mode. Anal. Methods 2012, 4, 4230–4236. [Google Scholar] [CrossRef]
- Akila, P.; Vennila, L. Chlorogenic acid a dietary polyphenol attenuates isoproterenol induced myocardial oxidative stress in rat myocardium: An in vivo study. Biomed. Pharmacother. 2016, 84, 208–214. [Google Scholar] [CrossRef]
- Akila, P.; Asaikumar, L.; Vennila, L. Chlorogenic acid ameliorates isoproterenol-induced myocardial injury in rats by stabilizing mitochondrial and lysosomal enzymes. Biomed. Pharmacother. 2017, 85, 582–591. [Google Scholar] [CrossRef]
- Kumaran, K.S.; Prince, P.S.M. Preventive effect of caffeic acid on lysosomal dysfunction in isoproterenol-induced myocardial infarcted rats. J. Biochem. Mol. Toxicol. 2010, 24, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Prince, P.S.M.; Kumaran, K.S. Preventive effects of caffeic acid on lipids, lipoproteins and glycoproteins in isoproterenol induced myocardial infarcted rats. Food Res. Int. 2012, 45, 155–160. [Google Scholar] [CrossRef]
- Park, J.B. 5-caffeoylquinic acid and caffeic acid orally administered suppress P-selectin expression on mouse platelets. J. Nutr. Biochem. 2009, 20, 800–805. [Google Scholar] [CrossRef]
- Dong, L.C.; Fan, Y.X.; Yu, Q.; Ma, J.; Dong, X.; Li, P.; Li, H.J. Synergistic effects of rhubarb-gardenia herb pair in cholestatic rats at pharmacodynamic and pharmacokinetic levels. J. Ethnopharmacol. 2015, 175, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Yao, H.; Shi, P.Y.; Huang, X.M.; Shen, Z.H.; Su, Y.; Yang, C.L.; Zhu, M.L.; Huang, L.Y.; Liu, A.L.; Lin, X.H. A strategy for integrated pharmacokinetic study of cardiovascular herbal medicines based on chemiluminescence and HPLC-MS/MS assays: A case using Danshen injection. RSC Adv. 2017, 7, 13570–13583. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Diplock, A.T. Current status of antioxidant therapy. Free Radic. Biol. Med. 1993, 15, 77–96. [Google Scholar] [CrossRef]
- Visioli, F.; Keaney, J.F.; Halliwell, B. Antioxidants and cardiovascular disease; panaceas or tonics for tired sheep? Cardiovasc. Res. 2000, 47, 409. [Google Scholar] [CrossRef] [Green Version]
- Plumb, G.W.; Garcia-Conesa, M.T.; Kroon, P.A.; Rhodes, M.; Ridley, S.; Williamson, G. Metabolism of chlorogenic acid by human plasma, liver, intestine and gut microflora. J. Sci. Food Agric. 1999, 79, 390–392. [Google Scholar] [CrossRef]
- Gonthier, M.P.; Verny, M.A.; Besson, C.; Remesy, C.; Scalbert, A. Chlorogenic acid bioavailability largely depends on its metabolism by the gut microflora in rats. J. Nutr. 2003, 133, 1853–1859. [Google Scholar] [CrossRef] [PubMed]
- Li, H.N.; Wang, S.W.; Yue, Z.H.; Ren, X.Q.; Xia, J.L. Traditional Chinese herbal injection: Current status and future perspectives. Fitoterapia 2018, 129, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.Y.; Lv, Y.N.; Fu, J.; Jia, Q.Q.; Han, S.L. A high expression Mas-related G protein coupled receptor X2 cell membrane chromatography coupled with liquid chromatography and mass spectrometry method for screening potential anaphylactoid components in kudiezi injection. J. Pharm. Biomed. Anal. 2018, 159, 483–489. [Google Scholar] [CrossRef] [PubMed]
Sample Availability: Samples of the caftaric acid, 5-CQA, chlorogenic acid, 4-CQA, caffeic acid, luteolin-7-O-β-d-gentiobioside, luteolin-7-O-β-d-glucuronide, luteolin-7-O-β-d-glucoside and apigenin-7-O-β-d-glucuronide are available from the authors. |






| Analyte | Regression Equation | Linearity Range (ng/mL) | R2 | LLOQ (ng/mL) |
|---|---|---|---|---|
| I | y = 0.39x + 0.00195 | 5–2500 | 0.9993 | 5 |
| II | y = 0.12x − 0.00323 | 10–5000 | 0.9995 | 10 |
| III | y = 0.94x + 0.00709 | 5–2500 | 0.9994 | 5 |
| IV | y = 0.45x + 0.00593 | 5–2500 | 0.9995 | 5 |
| V | y = 0.65x + 0.0138 | 5–2500 | 0.9984 | 5 |
| VI | y = 0.28x − 0.00115 | 5–2500 | 0.9989 | 5 |
| VII | y = 0.48x + 0.0180 | 5–2500 | 0.9974 | 5 |
| VIII | y = 0.62x + 0.0101 | 5–2500 | 0.9978 | 5 |
| IX | y = 0.47x + 0.0198 | 5–2500 | 0.9944 | 5 |
| Analyte | Concentration (ng/mL) | Intra-Day (n = 5) | Inter-Day (n = 5) | ||||
|---|---|---|---|---|---|---|---|
| Detected (ng/mL) | RSD (%) | Accuracy (RE, %) | Detected (ng/mL) | RSD (%) | Accuracy (RE, %) | ||
| I | 15 | 14.257 | 3.34 | −4.95 | 13.557 | 5.30 | −9.62 |
| 500 | 500.226 | 4.21 | 0.05 | 449.267 | 9.70 | −10.15 | |
| 2000 | 2129.731 | 2.80 | 6.49 | 1962.682 | 8.31 | −1.87 | |
| II | 30 | 27.386 | 7.17 | −8.71 | 28.735 | 12.57 | −4.22 |
| 1000 | 1102.605 | 3.02 | 10.26 | 950.930 | 12.61 | −4.91 | |
| 4000 | 4109.529 | 1.75 | 2.74 | 4066.613 | 2.37 | 1.67 | |
| III | 15 | 12.039 | 18.97 | −19.74 | 11.947 | 19.32 | −20.35 |
| 500 | 493.213 | 2.34 | −1.36 | 482.248 | 3.87 | −3.55 | |
| 2000 | 2041.617 | 3.64 | 2.08 | 2064.230 | 5.27 | 3.21 | |
| IV | 15 | 13.395 | 12.71 | −10.70 | 12.291 | 11.74 | −18.06 |
| 500 | 511.821 | 0.45 | 2.36 | 487.806 | 5.44 | −2.44 | |
| 2000 | 2132.444 | 4.23 | 6.62 | 2044.840 | 6.19 | 2.24 | |
| V | 15 | 12.376 | 10.20 | −17.49 | 12.074 | 12.55 | −19.51 |
| 500 | 492.319 | 1.74 | −1.54 | 450.435 | 14.46 | −9.91 | |
| 2000 | 2020.753 | 2.07 | 1.04 | 1780.322 | 15.39 | −10.98 | |
| VI | 15 | 13.368 | 11.23 | −10.88 | 15.466 | 20.49 | 3.10 |
| 500 | 471.679 | 5.01 | −5.66 | 439.213 | 8.93 | −12.16 | |
| 2000 | 1936.785 | 3.56 | −3.16 | 1915.598 | 6.16 | −4.22 | |
| VII | 15 | 14.459 | 15.81 | −3.61 | 14.403 | 18.32 | −3.98 |
| 500 | 491.497 | 4.83 | −1.70 | 449.924 | 9.11 | −10.02 | |
| 2000 | 1922.895 | 2.69 | −3.86 | 1826.993 | 6.82 | −8.65 | |
| VIII | 15 | 12.582 | 16.20 | −16.12 | 15.470 | 17.60 | 3.13 |
| 500 | 511.716 | 4.95 | 2.34 | 508.816 | 6.78 | 1.76 | |
| 2000 | 2046.100 | 2.81 | 2.30 | 2055.827 | 6.21 | 2.79 | |
| IX | 15 | 12.630 | 4.75 | −15.80 | 14.874 | 18.46 | −0.84 |
| 500 | 488.049 | 3.54 | −2.39 | 445.026 | 8.28 | −10.99 | |
| 2000 | 1758.415 | 4.41 | −12.08 | 1686.240 | 5.63 | −15.69 | |
| Analyte | Concentration (ng/mL) | Extraction Recoveries (n = 5) | Matrix Effects (n = 5) | ||
|---|---|---|---|---|---|
| Mean ± SD (%) | RSD (%) | Mean ± SD (%) | RSD (%) | ||
| I | 15 | 79.04 ± 12.01 | 15.20 | 86.47 ± 3.41 | 3.94 |
| 500 | 102.15 ± 11.92 | 11.67 | 87.12 ± 7.99 | 9.17 | |
| 2000 | 110.44 ± 7.70 | 6.97 | 80.66 ± 4.65 | 5.77 | |
| II | 30 | 77.25 ± 14.21 | 18.40 | >120% | 17.84 |
| 1000 | 93.55 ± 10.96 | 11.72 | >120% | 13.14 | |
| 4000 | 92.50 ± 9.94 | 10.75 | >120% | 2.98 | |
| III | 15 | 80.93 ± 12.44 | 15.38 | 97.01 ± 17.97 | 18.53 |
| 500 | 99.11 ± 14.40 | 14.52 | 97.56 ± 12.02 | 12.32 | |
| 2000 | 108.07 ± 9.19 | 8.50 | 98.09 ± 5.45 | 5.56 | |
| IV | 15 | 80.22 ± 13.97 | 17.42 | 96.01 ± 10.35 | 10.78 |
| 500 | 95.06 ± 10.15 | 10.68 | 112.54 ± 13.71 | 12.18 | |
| 2000 | 106.11 ± 13.71 | 12.92 | 109.26 ± 7.08 | 6.48 | |
| V | 15 | 96.72 ± 14.92 | 15.42 | 87.40 ± 7.05 | 8.06 |
| 500 | 104.88 ± 7.39 | 7.05 | 83.38 ± 5.72 | 6.86 | |
| 2000 | 118.98 ± 10.42 | 8.75 | 76.62 ± 5.68 | 7.41 | |
| VI | 15 | 71.81 ± 13.33 | 18.57 | 105.58 ± 18.19 | 17.23 |
| 500 | 79.45 ± 7.94 | 9.99 | 114.20 ± 7.96 | 6.97 | |
| 2000 | 80.38 ± 7.90 | 9.83 | 120.83 ± 7.51 | 6.22 | |
| VII | 15 | 80.69 ± 8.93 | 11.06 | 112.26 ± 6.54 | 5.82 |
| 500 | 81.63 ± 11.42 | 13.98 | 117.54 ± 7.13 | 6.07 | |
| 2000 | 90.05 ± 7.69 | 8.54 | 117.84 ± 8.99 | 7.63 | |
| VIII | 15 | 68.01 ± 10.65 | 15.65 | 104.51 ± 8.42 | 8.06 |
| 500 | 87.87 ± 5.70 | 6.48 | 107.99 ± 2.42 | 2.24 | |
| 2000 | 98.45 ± 8.54 | 8.67 | 113.43 ± 5.89 | 5.19 | |
| IX | 15 | 108.11 ± 16.38 | 15.15 | 104.55 ± 17.10 | 16.35 |
| 500 | 106.88 ± 13.60 | 12.72 | 111.22 ± 8.41 | 7.56 | |
| 2000 | 105.23 ± 9.64 | 9.16 | 109.25 ± 3.98 | 3.64 | |
| Analyte | Spiked (ng/mL) | 6 h in Auto-Sample | Freeze-Thaw Stability (Three Cycles) | Long-Term Stability (−80 °C, 14 Days) | |||
|---|---|---|---|---|---|---|---|
| RSD (%) | RE (%) | RSD (%) | RE (%) | RSD (%) | RE (%) | ||
| I | 15 | 9.43 | −15.03 | 3.59 | −16.92 | 10.78 | −10.71 |
| 500 | 4.47 | −14.93 | 8.33 | −8.67 | 2.88 | −1.12 | |
| 2000 | 8.69 | −5.26 | 4.79 | −2.66 | 4.79 | −3.42 | |
| II | 30 | 3.11 | −12.49 | 6.28 | −17.36 | 10.76 | 1.30 |
| 1000 | 4.94 | −9.25 | 6.92 | 2.66 | 4.22 | −5.34 | |
| 4000 | 2.81 | 3.63 | 1.42 | 3.72 | 5.60 | −10.05 | |
| III | 15 | 9.45 | −7.40 | 6.24 | −18.29 | 16.40 | −1.71 |
| 500 | 10.09 | −14.11 | 4.17 | −5.77 | 2.36 | −1.20 | |
| 2000 | 12.51 | −2.78 | 3.39 | 2.98 | 4.79 | 2.07 | |
| IV | 15 | 4.48 | −14.16 | 5.18 | −14.73 | 14.00 | −10.44 |
| 500 | 5.79 | −15.00 | 4.14 | −0.15 | 3.18 | −1.40 | |
| 2000 | 8.89 | 6.12 | 1.62 | 6.55 | 5.67 | 2.14 | |
| V | 15 | 5.96 | −18.04 | 8.47 | −13.66 | 10.02 | −17.21 |
| 500 | 6.18 | −14.10 | 5.15 | −2.10 | 5.63 | −5.99 | |
| 2000 | 13.85 | −11.88 | 5.58 | −7.76 | 2.65 | 7.83 | |
| VI | 15 | 7.10 | −14.11 | 5.94 | −13.29 | 8.77 | −0.68 |
| 500 | 2.16 | −14.25 | 10.28 | −3.78 | 0.89 | 2.31 | |
| 2000 | 6.52 | 0.23 | 7.58 | 9.34 | 6.84 | −5.24 | |
| VII | 15 | 10.02 | −7.74 | 10.41 | −10.80 | 6.63 | −2.91 |
| 500 | 6.91 | −12.46 | 4.02 | −1.08 | 1.86 | −0.26 | |
| 2000 | 6.73 | 5.65 | 5.59 | 9.76 | 5.75 | 8.01 | |
| VIII | 15 | 8.53 | −12.91 | 13.37 | −11.91 | 7.15 | −1.72 |
| 500 | 7.08 | −8.04 | 5.68 | 0.67 | 0.69 | 0.81 | |
| 2000 | 4.95 | 0.65 | 2.75 | 3.11 | 3.21 | 11.47 | |
| IX | 15 | 6.45 | −13.77 | 18.42 | −13.08 | 7.99 | −2.73 |
| 500 | 5.34 | −1.56 | 0.78 | 0.53 | 0.77 | −0.28 | |
| 2000 | 5.41 | 7.78 | 4.93 | 3.36 | 5.33 | 5.33 | |
| Analyte | t1/2 (h) | AUC0-t (h·ng/mL) | AUC0-∞ (h·ng/mL) | Vd (L/kg) | Cl (L/h/kg) | MRT0-t (h) | MRT0-∞ (h) |
|---|---|---|---|---|---|---|---|
| I | 2.22 ± 1.15 | 169.99 ± 55.71 | 209.20 ± 75.65 | 8.22 ± 2.51 | 2.99 ± 1.13 | 2.29 ± 1.65 | 3.94 ± 2.71 |
| II | 3.93 ± 0.47 | 2759.11 ± 399.66 | 3077.63 ± 482.58 | 22.10 ± 6.21 | 3.85 ± 0.62 | 3.84 ± 1.84 | 6.25 ± 2.83 |
| III | 3.36 ± 0.97 | 155.43 ± 23.39 | 199.84 ± 37.15 | 11.96 ± 3.10 | 2.53 ± 0.43 | 3.30 ± 0.99 | 5.93 ± 1.39 |
| IV | 2.92 ± 1.13 | 159.56 ± 39.37 | 209.31 ± 54.02 | 7.03 ± 1.29 | 1.81 ± 0.49 | 2.72 ± 1.32 | 5.07 ± 2.17 |
| V | 6.09 ± 0.90 | 145.82 ± 40.84 | 236.56 ± 43.58 | 17.92 ± 1.44 | 2.08 ± 0.42 | 4.09 ± 1.36 | 9.75 ± 1.70 |
| VI | 0.23 ± 0.12 | 163.85 ± 8.34 | 173.37 ± 14.97 | 2.51 ± 1.13 | 7.66 ± 0.71 | 0.20 ± 0.08 | 0.28 ± 0.15 |
| VII | 0.66 ± 0.20 | 671.81 ± 106.60 | 680.45 ± 108.60 | 5.30 ± 1.21 | 5.73 ± 0.97 | 0.25 ± 0.11 | 0.30 ± 0.12 |
| VIII | 0.13 ± 0.02 | 29.49 ± 2.38 | 31.01 ± 2.09 | 1.51 ± 0.35 | 8.25 ± 0.54 | 0.09 ± 0.02 | 0.11 ± 0.02 |
| IX | 0.07 ± 0.01 | 55.74 ± 8.50 | 56.11 ± 8.49 | 1.00 ± 0.24 | 10.07 ± 1.43 | 0.09 ± 0.01 | 0.09 ± 0.01 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, P.; Yang, C.; Su, Y.; Huang, L.; Lin, X.; Yao, H. Simultaneous Determination of Five Phenolic Acids and Four Flavonoid Glycosides in Rat Plasma Using HPLC-MS/MS and Its Application to a Pharmacokinetic Study after a Single Intravenous Administration of Kudiezi Injection. Molecules 2019, 24, 64. https://doi.org/10.3390/molecules24010064
Shi P, Yang C, Su Y, Huang L, Lin X, Yao H. Simultaneous Determination of Five Phenolic Acids and Four Flavonoid Glycosides in Rat Plasma Using HPLC-MS/MS and Its Application to a Pharmacokinetic Study after a Single Intravenous Administration of Kudiezi Injection. Molecules. 2019; 24(1):64. https://doi.org/10.3390/molecules24010064
Chicago/Turabian StyleShi, Peiying, Chunlei Yang, Ya Su, Liying Huang, Xinhua Lin, and Hong Yao. 2019. "Simultaneous Determination of Five Phenolic Acids and Four Flavonoid Glycosides in Rat Plasma Using HPLC-MS/MS and Its Application to a Pharmacokinetic Study after a Single Intravenous Administration of Kudiezi Injection" Molecules 24, no. 1: 64. https://doi.org/10.3390/molecules24010064
APA StyleShi, P., Yang, C., Su, Y., Huang, L., Lin, X., & Yao, H. (2019). Simultaneous Determination of Five Phenolic Acids and Four Flavonoid Glycosides in Rat Plasma Using HPLC-MS/MS and Its Application to a Pharmacokinetic Study after a Single Intravenous Administration of Kudiezi Injection. Molecules, 24(1), 64. https://doi.org/10.3390/molecules24010064