Wine Fining with Plant Proteins
Abstract
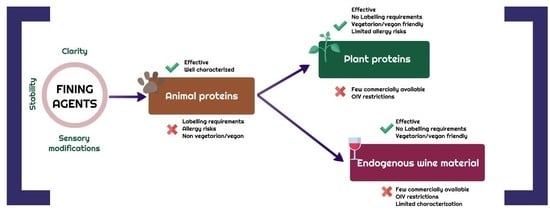
:1. Introduction
2. Wine Fining with Plant Proteins
2.1. Wine Fining with Cereal-Based Proteins
2.1.1. Gluten
2.1.2. Maize Zeins and Rice Proteins
2.2. Wine Fining with Legume-Based Proteins
2.3. Wine Fining with Potato Proteins
2.4. Wine Fining with Grape Seed Extract (GSE)
3. How to Avoid the Allergenic Risk Deriving from Exogenous Protein Residues in Wines?
4. Development of Novel Plant Protein Fining Agents
- Sourcing the material for protein extraction. The development of a new fining agent needs to begin with the selection of the starting material (protein source) from which extracting the proteins. Several considerations can be made to inform this decision. Ideally, the source should be rich in proteins, and could be both an intact plant organ (e.g., seeds, fruits, roots etc.), or a by-product resulting from an industrial plant processing. Several examples have been previously discussed, including by-products of the oil (e.g., the grape seed flour [15]) and of the starch (e.g., maize zeins [8], rice proteins [49]) industries. Ideally, the selected material should be inexpensive and readily available, because otherwise the products, even if shown to be effective, would be inappropriate for large scale distribution. Additionally, the starting material should be allergen free, or at least not subjected to allergenic labeling [45], safe for consumption, and containing no or very low quantities of compounds that would potentially impact the wine sensory attributes (e.g., off flavors, colored material, phenolics, etc.).
- Extraction methods requirements. Proteins can be extracted from plant materials with a variety of methods, but in this case protein extraction needs to be performed only with the use of food grade and inexpensive reagents, and should include only a limited number of steps in order to be as simple as possible and consequently economically viable.
- Preparation of the extracts. The different extraction methods theoretically designed, also considering the solubility properties of the protein fractions of interest, will need to be tested for protein content (e.g., by nitrogen quantification or colorimetric methods) in order to obtain the highest yield. Based on the extraction yield, the best performing method is then selected. The liquid extracts obtained can be kept in a liquid form when the extracted proteins are soluble, or the proteins can be partially purified (e.g., by precipitation via ethanol addition or pH changes), but the purification steps should be reduced at the minimum due to their additional direct (e.g., reagents, equipment, time, labor), and indirect (e.g., loss in protein yield) costs. A preliminary fining test (see next point) at this stage can be done to inform about the potential efficacy of the extract. Proteins can also be modified to improve their binding ability. These modifications generally include protein thermal denaturation [8], partial hydrolysis, and use of food grade reducing agents (e.g., sulfites). All these treatments are performed to modify the size and number/accessibility of the tannin binding sites, so to favor the interactions with wine phenolics compounds [6,77].
- Testing the fining efficacy of the selected extract. The fining ability of the obtained proteinaceous extract(s) will need to be assessed on un-fined musts and/or wines. Preliminary fining trials should be done using different dosages of the fining agent at laboratory scale (e.g., 100–200 mL of must or wine). To this aim, the chosen dosages will depend on the protein content of the extract, but generally a preliminary trial could look at addition rates of 0, 1, 5, 10, 20 and 40 g/hL. If the extract is in a solid form, it is recommended to prepare a concentrated stock solution by dissolving/dispersing it in a small quantity of liquid (e.g., water, must or wine). The fining agent will then be added to the must or wine in a graduated cylinder and mixed well. For the assessment of chemical and sensory impact of the treatment, the wine can be let in contact with the fining agent for at least 24 h, while longer periods (e.g., 5 days) are needed to evaluate its clarification ability. During the contact period the volume of the precipitated material will be recorded to have information on how much wine is trapped on the lees [78] and on the compactness of the deposit, as this can affect the wine racking and/or filtration efficiencies. Variations in turbidity of the wine as a function of the different dosages used will also be recorded by nephelometry. This allows to have an idea of the time required to reach satisfactory wine clarification for the different doses. The wine will then be separated from the insoluble material by centrifugation (e.g., >3000 g) and/or filtration (e.g., glass fiber filter followed by 0.45 µm), and analyzed in comparison with the un-fined control sample (0 g/L fining agent). Wine chemical characterization should be performed according to the OIV official methods of analysis of reference [79], and should include basic wine parameters (e.g., pH, ethanol content, titratable acidity), chromatic characteristics, oxidative stability, phenolic content (e.g., by Folin-Ciocalteau method), and if more information is needed additional analysis as phenolic profile including anthocyanins for reds (by HPLC) [50,73], astringency assessments (e.g., mucin index [65]), variation in sensory attributes (e.g., astringency, bitterness, aroma [4,9]), and volatile composition by GC [10] can be done. Additionally, in case the starting plant material is already included in the list of the allergens, tests for checking the presence of protein residues (e.g., by immunological or MS-based methods) in the filtered wines treated with different doses are also necessary [80].
- Benchmarking versus commercial products. When a full analytical picture is obtained, the best fining addition rate(s) should be selected, and the experiment should be replicated benchmarking the new product with other commercially available fining agents. At times the benchmarking can be done simultaneously with the previous step, although this approach could be more time consuming. To gain information on the general effects of the new preparation, different wine styles should be tested, including at least one white and one red wine. For each wine, the effects must be considered in relation to the desired aims (e.g., clarification, stabilization, modification of the sensory properties) as they can be different depending on the wine characteristics. In this context, it could advisable to use the fining agents that are commonly employed for a specific type of wine as a benchmark.
- Scale up. Once the benchmarking has given satisfactory results, the experiment should be scaled up in terms of volume of wine treated (for example 100 L or more) to allow for more complex sensory tests requiring an amount of wine higher than that of the laboratory scale trials (e.g., descriptive sensory analysis and preference tests) and also to confirm the results obtained by these trials.
- Development of a commercial product. The product will need to be checked for stability, and its shelf life and appropriate storage conditions determined. Finally, authorization for use in winemaking will need to be requested to the appropriate governing bodies (e.g., the European Union, OIV).
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Waterhouse, A.L.; Sacks, G.L.; Jeffery, D.W. Understanding Wine Chemistry, 1st ed.; John Wiley & Sons: Hoboken, NJ, USA, 2016. [Google Scholar]
- Oberholster, A.; Carstens, L.M.; du Toit, W.J. Investigation of the effect of gelatine, egg albumin and cross-flow microfiltration on the phenolic composition of Pinotage wine. Food Chem. 2013, 138, 1275–1281. [Google Scholar] [CrossRef]
- Hrazdina, G.; Van Buren, J.P.; Robinson, W.B. Influence of molecular size of gelatin on reaction with tannic acid. Am. J. Enol. Vitic. 1969, 20, 66–68. [Google Scholar]
- Sanborn, M.; Edwards, C.G.; Ross, C.F. Impact of fining on chemical and sensory properties of Washington State Chardonnay and Gewürztraminer wines. Am. J. Enol. Vitic. 2010, 61, 31–41. [Google Scholar]
- Iturmendi, N.; Durán, D.; Marín-Arroyo, M.R. Fining of red wines with gluten or yeast extract protein. Int. J. Food Sci. Technol. 2010, 45, 200–207. [Google Scholar] [CrossRef]
- Marchal, R.; Marchal-Delahaut, L.; Michels, F.; Parmentier, M.; Lallement, A.; Jeandet, P. Use of wheat gluten as clarifying agent of musts and white wines. Am. J. Enol. Vitic. 2002, 53, 308–314. [Google Scholar]
- Simonato, B.; Mainente, F.; Tolin, S.; Pasini, G. Immunochemical and mass spectrometry detection of residual proteins in gluten fined red wine. J. Agric. Food Chem. 2011, 59, 3101–3110. [Google Scholar] [CrossRef]
- Simonato, B.; Mainente, F.; Suglia, I.; Curioni, A.; Pasini, G. Evaluation of fining efficiency of corn zeins in red wine: a preliminary study. Ital. J. Food Sci. 2009, 21, 97–105. [Google Scholar]
- Simonato, B.; Mainente, F.; Selvatico, E.; Violoni, M.; Pasini, G. Assessment of the fining efficiency of zeins extracted from commercial corn gluten and sensory analysis of the treated wine. LWT-Food Sci. Technol. 2013, 54, 549–556. [Google Scholar] [CrossRef]
- Granato, T.M.; Ferranti, P.; Iametti, S.; Bonomi, F. Affinity and selectivity of plant proteins for red wine components relevant to color and aroma traits. Food Chem. 2018, 256, 235–243. [Google Scholar] [CrossRef]
- Gambuti, A.; Rinaldi, A.; Moio, L. Use of patatin, a protein extracted from potato, as alternative to animal proteins in fining of red wine. Eur. Food Res. Technol. 2012, 235, 753–765. [Google Scholar] [CrossRef]
- Gambuti, A.; Rinaldi, A.; Romano, R.; Manzo, N.; Moio, L. Performance of a protein extracted from potatoes for fining of white musts. Food Chem. 2016, 190, 237–243. [Google Scholar] [CrossRef] [Green Version]
- Noriega-Domínguez, M.J.; Durán, D.S.; Vírseda, P.; Marín-Arroyo, M.R. Non-animal proteins as clarifying agents for red wines. J. Int. des Sci. la vigne du vin 2010, 44, 179. [Google Scholar] [CrossRef]
- Gazzola, D.; Vincenzi, S.; Marangon, M.; Pasini, G.; Curioni, A. Grape seed extract: the first protein-based fining agent endogenous to grapes. Aust. J. Grape Wine Res. 2017, 23. [Google Scholar] [CrossRef]
- Vincenzi, S.; Dinnella, C.; Recchia, A.; Monteleone, E.; Gazzola, D.; Pasini, G.; Curioni, A. Grape seed proteins: A new fining agent for astringency reduction in red wine. Aust. J. Grape Wine Res. 2013, 19, 153–160. [Google Scholar] [CrossRef]
- Marangon, M.; Nesbitt, A.; Milanowski, T. Global climate change and wine safety. In Wine Safety, Consumer Preference, and Human Health; Springer International Publishing: Berlin, Germany, 2016; pp. 97–116. [Google Scholar]
- Maury, C.; Sarni-Manchado, P.; Lefebvre, S.; Cheynier, V.; Moutounet, M. Influence of fining with different molecular weight gelatins on proanthocyanidin composition and perception of wines. Am. J. Enol. Vitic. 2001, 52, 140–145. [Google Scholar]
- Sarni-Manchado, P.; Deleris, A.; Avallone, S.; Cheynier, V.; Moutounet, M. Analysis and characterization of wine condensed tannins precipitated by proteins used as fining agent in enology. Am. J. Enol. Vitic. 1999, 50, 81–86. [Google Scholar]
- Smith, P.A.; McRae, J.M.; Bindon, K.A. Impact of winemaking practices on the concentration and composition of tannins in red wine. Aust. J. Grape Wine Res. 2015, 21, 601–614. [Google Scholar] [CrossRef]
- Tschiersch, C.; Nikfardjam, M.P.; Schmidt, O.; Schwack, W. Degree of hydrolysis of some vegetable proteins used as fining agents and its influence on polyphenol removal from red wine. Eur. Food Res. Technol. 2010, 231, 65–74. [Google Scholar] [CrossRef]
- Deckwart, M.; Carstens, C.; Webber-Witt, M.; Schäfer, V.; Eichhorn, L.; Schröter, F.; Fischer, M.; Brockow, K.; Christmann, M.; Paschke-Kratzin, A. Impact of wine manufacturing practice on the occurrence of fining agents with allergenic potential. Food Addit. Contam. Part A 2014, 31, 1805–1817. [Google Scholar] [CrossRef]
- Stockley, C.S.; Johnson, D.L. Adverse food reactions from consuming wine. Aust. J. Grape Wine Res. 2015, 21, 568–581. [Google Scholar] [CrossRef]
- Tolin, S.; Pasini, G.; Simonato, B.; Mainente, F.; Arrigoni, G. Analysis of commercial wines by LC-MS/MS reveals the presence of residual milk and egg white allergens. Food Control 2012, 28, 321–326. [Google Scholar] [CrossRef]
- Weber, P.; Steinhart, H.; Paschke, A. Investigation of the allergenic potential of wines fined with various proteinogenic fining agents by ELISA. J. Agric. Food Chem. 2007, 55, 3127–3133. [Google Scholar] [CrossRef]
- European Commission Commission Implementing Regulation (EU) No 579/2012 of 29 June 2012 amending Regulation (EC) No. 607/2009 laying down certain detailed rules for the implementation of Council Regulation (EC) No. 479/2008 as regards protected designations of origin and geo. Off. J. Eur. Union 2012, 171, 4–7.
- Marchal, R.; Lallement, A.; Jeandet, P.; Establet, G. Clarification of Muscat musts using wheat proteins and the flotation technique. J. Agric. Chem. Environ. 2003, 51, 2040–2048. [Google Scholar] [CrossRef]
- Marchal, R.; Marchal-Delahaut, L.; Lallement, A.; Jeandet, P. Wheat gluten used as a clarifying agent of red wines. J. Agric. Food Chem. 2001, 50, 177–184. [Google Scholar] [CrossRef]
- Maury, C.; Sarni-Manchado, P.; Lefebvre, S.; Cheynier, V.; Moutounet, M. Influence of fining with plant proteins on proanthocyanidin composition of red wines. Am. J. Enol. Vitic. 2003, 33, 105–111. [Google Scholar]
- María Remedios, M.-A.; Iturmendi, N.; Noriega, M.J. Gluten and yeast extract protein: New fining agents for red wines. Agro Food Ind. Hi. Tech. 2010, 21, 29–32. [Google Scholar]
- Granato, T.M.; Nasi, A.; Ferranti, P.; Iametti, S.; Bonomi, F. Fining white wine with plant proteins: effects of fining on proanthocyanidins and aroma components. Eur. Food Res. Technol. 2014, 238, 265–274. [Google Scholar] [CrossRef]
- Maury, C.; Sarni-Manchado, P.; Cheynier, V. Highlighting protein fining residues in a model red wine. Food Chem. 2019, 279, 272–278. [Google Scholar] [CrossRef]
- Cosme, F.; Ricardo-Da-Silva, J.M.; Laureano, O. Protein fining agents: Characterization and red wine fining assays. Ital. J. Food Sci. 2007, 19, 39–56. [Google Scholar]
- González-Neves, G.; Favre, G.; Gil, G. Effect of fining on the colour and pigment composition of young red wines. Food Chem. 2014, 157, 385–392. [Google Scholar] [CrossRef]
- Fernandes, J.P.; Neto, R.; Centeno, F.; De Fátima Teixeira, M.; Gomes, A.C. Unveiling the potential of novel yeast protein extracts in white wines clarification and stabilization. Front. Chem. 2015, 3. [Google Scholar] [CrossRef] [Green Version]
- Shewry, P.R.; Tatham, A.S. Disulphide bonds in wheat gluten proteins. J. Cereal Sci. 1997, 25, 207–227. [Google Scholar] [CrossRef]
- Simonato, B.; De Lazzari, F.; Pasini, G.; Polato, F.; Giannattasio, M.; Gemignani, C.; Peruffo, A.D.; Santucci, B.; Plebani, M.; Curioni, A. IgE binding to soluble and insoluble wheat flour proteins in atopic and non-atopic patients suffering from gastrointestinal symptoms after wheat ingestion. Clin. Exp. Allergy 2001, 31, 1771–1778. [Google Scholar] [CrossRef]
- Koning, F. Adverse effects of wheat gluten. Ann. Nutr. Metab. 2015, 67, 7–14. [Google Scholar] [CrossRef]
- Igbinedion, S.O.; Ansari, J.; Vasikaran, A.; Gavins, F.N.; Jordan, P.; Boktor, M.; Alexander, J.S. Non-celiac gluten sensitivity: All wheat attack is not celiac. World J. Gastroenterol. 2017, 23, 7201–7210. [Google Scholar] [CrossRef]
- EFSA NDA Panel. Scientific Opinion on the evaluation of allergenic foods and food ingredients for labeling purposes. EFSA J. 2014, 12, 3894. [Google Scholar]
- EFSA NDA Panel. Opinion of the Scientific Panel on Dietetic Products, Nutrition and Allergies on a request from the Commission related to a notification from SOFRALAB on hydrolysed wheat gluten used as fining agent in wines pursuant to Article 6 Paragraph 11 of Directive. EFSA J. 2004, 148, 1–8. [Google Scholar]
- Cattaneo, A.; Ballabio, C.; Bernardini, R.; Bertelli, A.A.E.; Novembre, E.; Vierucci, A.; Restani, P. Assessment of residual immunoreactivity in red or white wines clarified with pea or lupin extracts. Int. J. Tissue React. 2003, 25, 159–165. [Google Scholar]
- Cattaneo, A.; Ballabio, C.; Bertelli, A.A.; Fiocchi, A.; Galli, C.L.; Isoardi, P.; Terracciano, L.; Restani, P. Evaluation of residual immunoreactivity in red and white wines clarified with gluten or gluten derivatives. Int. J. Tissue React. 2003, 25, 57–64. [Google Scholar]
- OIV Resolution OIV-OENO 28/2004: Codex—Protein Plant Origin. 2004, pp. 1–5. Available online: http://www.oiv.int/en/technical-standards-and-documents/resolutions-of-the-oiv/oenology-resolutions (accessed on 8 June 2019).
- European Commission. Directive 2003/89/EC of the European Parliament and of the Council of 10 November 2003 amending Directive 2000/13/EC as regards indication of the ingredients present in foodstuffs. Off. J. Eur. Union 2003, 308, 15–18. [Google Scholar]
- European Commission. Directive 2007/68/EC of 27 November 2007 amending Annex IIIa of the Directive 2001/13/EC of the European Parliament and of the Council as regards certain food ingredients. Off. J. Eur. Union 2007, 310, 15–18. [Google Scholar]
- Pasini, G.; Simonato, B.; Curioni, A.; Vincenzi, S.; Cristaudo, A.; Santucci, B.; Peruffo, A.D.B.; Giannattasio, M. IgE-mediated allergy to corn: a 50 kDa protein, belonging to the reduced soluble proteins, is a major allergen. Allergy 2002, 57, 98–106. [Google Scholar] [CrossRef]
- Shih, F.F.; Daigle, K.W. Preparation and characterization of rice protein isolates. J. Am. Oil Chem. Soc. 2000, 77, 885–889. [Google Scholar] [CrossRef]
- Mira, H.; Leite, P.; Ricardo-Da-Silva, J.M.; Curvelo-Garcia, A.S. Plant proteins in wine fining: influence on chemical and sensory characteristics. Le Bull. l’OIV 2006, 79, 277–295. [Google Scholar]
- Gazzola, D.; Vincenzi, S.; Lamborghini, M.; Pasini, G.; Curioni, A. Rice protein extracts as wine fining agents. In Proceedings of the Macrowine 2014: Macromolecules and Secondary Metabolites of Grapevine and Wines, Stellenbosch, South Africa, 7–10 September 2014; p. 99. [Google Scholar]
- Kang, W.; Niimi, J.; Bastian, S.E.P. Reduction of red wine astringency perception using vegetable protein fining agents. Am. J. Enol. Vitic. 2018, 69, 22–31. [Google Scholar] [CrossRef]
- Cosme, F.; Capão, I.; Filipe-Ribeiro, L.; Bennett, R.N.; Mendes-Faia, A. Evaluating potential alternatives to potassium caseinate for white wine fining: Effects on physicochemical and sensory characteristics. LWT-Food Sci. Technol. 2012, 46, 382–387. [Google Scholar] [CrossRef]
- Jensen, L.B.; Pedersen, M.H.; Skov, P.S.; Poulsen, L.K.; Bindslev-Jensen, C.; Andersen, S.B.; Torp, A.M. Peanut cross-reacting allergens in seeds and sprouts of a range of legumes. Clin. Exp. Allergy 2008, 38, 1969–1977. [Google Scholar] [CrossRef]
- Verma, A.K.; Kumar, S.; Das, M.; Dwivedi, P.D. A comprehensive review of legume allergy. Clin. Rev. Allergy Immunol. 2013, 45, 30–46. [Google Scholar] [CrossRef]
- Vereda, A.; Van Hage, M.; Ahlstedt, S.; Ibañez, M.D.; Cuesta-Herranz, J.; Van Odijk, J.; Wickman, M.; Sampson, H.A. Peanut allergy: Clinical and immunologic differences among patients from 3 different geographic regions. J. Allergy Clin. Immunol. 2011, 127, 603–607. [Google Scholar] [CrossRef]
- Knorr, D.; Kohler, G.O.; Betschart, A.A. Potato protein concentrates: the influence of various methods of recovery upon yield, compositional and functional characteristics. J. Food Process. Preserv. 1977, 1, 235–247. [Google Scholar] [CrossRef]
- Bártová, V.; Bárta, J. Chemical composition and nutritional value of protein concentrates isolated from potato (Solanum tuberosum L.) fruit juice by precipitation with ethanol or ferric chloride. J. Agric. Food Chem. 2009, 57, 9028–9034. [Google Scholar] [CrossRef]
- Chiriac, A.M.; Bourrain, J.L.; Lepicard, E.; Molinari, N.; Demoly, P. Prevalence of sensitization and allergy to potato in a large population. J. Allergy Clin. Immunol. Pract. 2017, 5, 507–509. [Google Scholar] [CrossRef]
- Schmidt, M.H.H.; Raulf-Heimsoth, M.; Posch, A. Evaluation of patatin as a major cross-reactive allergen in latex-induced potato allergy. Ann. Allergy, Asthma Immunol. 2002, 89, 613–618. [Google Scholar] [CrossRef]
- OIV Resolution OIV-OENO 495-2013: Monograph on Protein Plant Origin—Modification of the File. 2013, p. 1. Available online: http://www.oiv.int/en/technical-standards-and-documents/resolutions-of-the-oiv/oenology-resolutions (accessed on 8 June 2019).
- Bautista-Ortín, A.B.; Ruiz-García, Y.; Marín, F.; Molero, N.; Apolinar-Valiente, R.; Gómez-Plaza, E. Remarkable proanthocyanidin adsorption properties of monastrell pomace cell wall material highlight its potential use as an alternative fining agent in red wine production. J. Agric. Food Chem. 2015, 63, 620–633. [Google Scholar] [CrossRef]
- Jiménez-Martínez, M.D.; Gómez-Plaza, E.; Molero, N.; Bautista-Ortín, A.B. Fining of red wines with pomace cell wall material: effect on wine phenolic composition. Food Bioprocess Technol. 2017, 10, 1531–1539. [Google Scholar] [CrossRef]
- Lochbühler, B.; Manteau, S.; Morge, C.; Caillet, M.-M.; Charpentier, C.; Schnell, S.; Grossmann, M.; Rauhut, D. Yeast protein extracts: an alternative fining agent for red wines. Eur. Food Res. Technol. 2015, 240, 689–699. [Google Scholar] [CrossRef]
- Nguela, J.M.; Poncet-Legrand, C.; Sieczkowski, N.; Vernhet, A. Interactions of grape tannins and wine polyphenols with a yeast protein extract, mannoproteins and β-glucan. Food Chem. 2016, 210, 671–682. [Google Scholar] [CrossRef]
- Curioni, A.; Vincenzi, S. Grape Seed Extracts, Method of Preparation and Use Thereof for Treating Wines. EP2404509A1, 11 January 2012. [Google Scholar]
- Monteleone, E.; Condelli, N.; Dinnella, C.; Bertuccioli, M. Prediction of perceived astringency induced by phenolic compounds. Food Qual. Prefer. 2004, 15, 761–769. [Google Scholar] [CrossRef]
- OIV Resolution OIV/Oeno 427/2010: Criteria for the Methods of Quantification of Potentially Allergenic Residues of Fining Agent Proteins in Wine. 2010, p. 1. Available online: http://www.oiv.int/en/technical-standards-and-documents/resolutions-of-the-oiv/oenology-resolutions (accessed on 8 June 2019).
- OIV Resolution OIV-OENO 520-2014: Code of Good Fining Practices for Wine to Be Applied in the Use of Proteinaceous Wine Fining Agents with Allergenic Potential (Casein and Egg White). 2014, pp. 1–6. Available online: http://www.oiv.int/en/technical-standards-and-documents/resolutions-of-the-oiv/oenology-resolutions (accessed on 8 June 2019).
- Weber, P.; Steinhart, H.; Paschke, A. Determination of the bovine food allergen casein in white wines by quantitative indirect ELISA, SDS−PAGE, western blot and immunostaining. J. Agric. Food Chem. 2009, 57, 8399–8405. [Google Scholar] [CrossRef]
- Sauvage, F.-X.; Bach, B.; Moutounet, M.; Vernhet, A. Proteins in white wines: Thermo-sensitivity and differential adsorbtion by bentonite. Food Chem. 2010, 118, 26–34. [Google Scholar] [CrossRef]
- Weber, P.; Steinhart, H.; Paschke, A. Characterization, antigenicity and detection of fish gelatine and isinglass used as processing aids in wines. Food Addit. Contam. Part A 2010, 27, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Renard, C.M.G.; Baron, A.; Guyot, S.; Drilleau, J.-F. Interactions between apple cell walls and native apple polyphenols: quantification and some consequences. Int. J. Biol. Macromol. 2001, 29, 115–125. [Google Scholar] [CrossRef]
- Bindon, K.A.; Smith, P.A. Comparison of the affinity and selectivity of insoluble fibres and commercial proteins for wine proanthocyanidins. Food Chem. 2013, 136, 917–928. [Google Scholar] [CrossRef]
- Guerrero, R.F.; Smith, P.; Bindon, K.A. Application of insoluble fibers in the fining of wine phenolics. J. Agric. Food Chem. 2013, 61, 4424–4432. [Google Scholar] [CrossRef]
- Jiménez-Martínez, M.D.; Gil-Muñoz, R.; Gómez-Plaza, E.; Bautista-Ortín, A.B. Performance of purified grape pomace as a fining agent to reduce the levels of some contaminants from wine. Food Addit. Contam. Part A 2018, 35, 1061–1070. [Google Scholar] [CrossRef]
- Gil, M.; Del Barrio-Galán, R.; Úbeda, C.; Peña-Neira, Á. Effectiveness of fibers from “Cabernet Sauvignon” (Vitis vinifera) pomace as fining agents for red aines. J. Food Qual. 2018, 2018, 1–13. [Google Scholar] [CrossRef]
- Jiménez-Martínez, M.D.; Bautista-Ortín, A.B.; Gil-Muñoz, R.; Gómez-Plaza, E. Fining with purified grape pomace. Effect of dose, contact time and varietal origin on the final wine phenolic composition. Food Chem. 2019, 271, 570–576. [Google Scholar] [CrossRef]
- Granato, T.M.; Piano, F.; Nasi, A.; Ferranti, P.; Iametti, S.; Bonomi, F. Molecular basis of the interaction between proteins of plant origin and proanthocyanidins in a model wine system. J. Agric. Food Chem. 2010, 58, 11969–11976. [Google Scholar] [CrossRef]
- Salazar, F.N.; Marangon, M.; Labbé, M.; Lira, E.; Rodríguez-Bencomo, J.J.; López, F. Comparative study of sodium bentonite and sodium-activated bentonite fining during white wine fermentation: its effect on protein content, protein stability, lees volume, and volatile compounds. Eur. Food Res. Technol. 2017, 243. [Google Scholar] [CrossRef]
- OIV—International Organisation of Vine and Wine. Compendium of International Methods of Analysis of Wines and Musts. Available online: http://www.oiv.int/en/technical-standards-and-documents/methods-of-analysis/compendium-of-international-methods-of-analysis-of-wines-and-musts-2-vol (accessed on 8 June 2019).
- Rizzi, C.; Mainente, F.; Pasini, G.; Simonato, B. Hidden exogenous proteins in wine: problems, methods of detection and related legislation—A review. Czech J. Food Sci. 2016, 34, 93–104. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marangon, M.; Vincenzi, S.; Curioni, A. Wine Fining with Plant Proteins. Molecules 2019, 24, 2186. https://doi.org/10.3390/molecules24112186
Marangon M, Vincenzi S, Curioni A. Wine Fining with Plant Proteins. Molecules. 2019; 24(11):2186. https://doi.org/10.3390/molecules24112186
Chicago/Turabian StyleMarangon, Matteo, Simone Vincenzi, and Andrea Curioni. 2019. "Wine Fining with Plant Proteins" Molecules 24, no. 11: 2186. https://doi.org/10.3390/molecules24112186
APA StyleMarangon, M., Vincenzi, S., & Curioni, A. (2019). Wine Fining with Plant Proteins. Molecules, 24(11), 2186. https://doi.org/10.3390/molecules24112186