Natural Polysaccharides for siRNA Delivery: Nanocarriers Based on Chitosan, Hyaluronic Acid, and Their Derivatives
Abstract
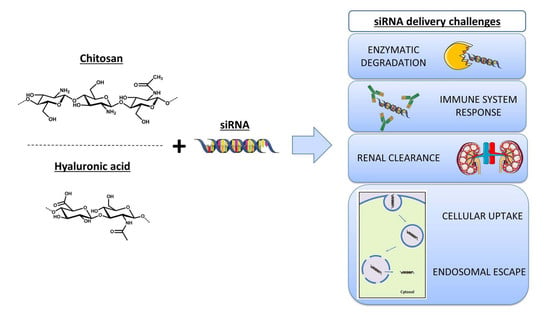
:1. RNA Interference for Gene Silencing
2. Use of Natural Polysaccharides as Nanocarriers for Gene Silencing
2.1. Polyelectrolyte Complexes
2.2. Nanogels
2.3. Polymeric Micelles
3. Chitosan for siRNA Delivery
3.1. Factors Affecting Gene Silencing Efficiency
3.2. Applications of Native Chitosan-Based Nanocarriers
3.3. Modifications to Improve the Efficiency of Chitosan-Based siRNA Nanocarriers
3.3.1. Chitosan Solubility and Delivery System Stability
3.3.2. Cellular Uptake and Endosomal Escape
3.3.3. Cell Targeting Specificity and Biodistribution
3.3.4. Co-Delivery of siRNA and Pharmaceuticals
4. Hyaluronic Acid for siRNA Delivery
4.1. Factors Affecting Gene Silencing Efficiency and Applications of Native Hyaluronic Acid-Based Nanocarriers
4.2. Modifications to Improve the Efficiency of Hyaluronic Acid-Based siRNA Nanocarriers
4.2.1. siRNA Loading and Stability
4.2.2. Cell Targeting Specificity and Biodistribution
4.2.3. Co-Delivery of siRNA and Pharmaceuticals
5. siRNA Nanocarriers Combining Chitosan and Hyaluronic Acid
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Elbashir, S.M.; Harborth, J.; Lendeckel, W.; Yalcin, A.; Weber, K.; Tuschl, T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 2001, 411, 494–498. [Google Scholar] [CrossRef] [PubMed]
- Mokhtarzadeh, A.; Alibakhshi, A.; Hashemi, M.; Hejazi, M.; Hosseini, V.; de la Guardia, M.; Ramezani, M. Biodegradable nano-polymers as delivery vehicles for therapeutic small non-coding ribonucleic acids. J. Control. Release 2017, 245, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Snead, N.M.; Rossi, J.J. Biogenesis and function of endogenous and exogenous siRNAs. Wiley Interdiscip. Rev. RNA 2010, 1, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Jackson, A.L.; Linsley, P.S. Recognizing and avoiding siRNA off-target effects for target identification and therapeutic application. Nat. Rev. Drug Discov. 2010, 9, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Piatek, M.J.; Werner, A. Endogenous siRNAs: Regulators of internal affairs. Biochem. Soc. Trans. 2014, 42, 1174–1179. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Joyce, C.E.; Bowcock, A.M.; Zhang, W. Noncanonical microRNAs and endogenous siRNAs in normal and psoriatic human skin. Hum. Mol. Genet. 2013, 22, 737–748. [Google Scholar] [CrossRef]
- Whitehead, K.A.; Langer, R.; Anderson, D.G. Knocking down barriers: Advances in siRNA delivery. Nat. Rev. Drug Discov. 2009, 8, 129–138. [Google Scholar] [CrossRef]
- Jeang, K.-T. RNAi in the regulation of mammalian viral infections. BMC Biol. 2012, 10, 58. [Google Scholar] [CrossRef]
- Cavallaro, G.; Sardo, C.; Craparo, E.F.; Porsio, B.; Giammona, G. Polymeric nanoparticles for siRNA delivery: Production and applications. Int. J. Pharm. 2017, 525, 313–333. [Google Scholar] [CrossRef]
- Kanasty, R.; Dorkin, J.R.; Vegas, A.; Anderson, D. Delivery materials for siRNA therapeutics. Nat. Mater. 2013, 12, 967–977. [Google Scholar] [CrossRef] [PubMed]
- Tamura, A.; Nagasaki, Y. Smart siRNA delivery systems based on polymeric nanoassemblies and nanoparticles. Nanomedicine 2010, 5, 1089–1102. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, N.; Dasaradhi, P.V.N.; Mohmmed, A.; Malhotra, P.; Bhatnagar, R.K.; Mukherjee, S.K. RNA interference: Biology, mechanism, and applications. Microbiol. Mol. Biol. Rev. 2003, 67, 657–685. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.H.; Revuri, V.; Lee, S.-J.J.; Cho, S.; Park, I.-K.K.; Cho, K.J.; Bae, W.K.; Lee, Y.K. Oral siRNA Delivery to Treat Colorectal Liver Metastases. ACS Nano 2017, 11, 10417–10429. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.K.; Yu, X.F.; Wang, J.H.; Li, Z.B.; Li, P.H.; Wang, H.; Song, L.; Chu, P.K.; Li, C. Gold-nanorods-siRNA nanoplex for improved photothermal therapy by gene silencing. Biomaterials 2016, 78, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Aagaard, L.; Rossi, J.J. RNAi therapeutics: Principles, prospects and challenges. Adv. Drug Deliv. Rev. 2007, 59, 75–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iranpur Mobarakeh, V.; Modarressi, M.H.; Rahimi, P.; Bolhassani, A.; Arefian, E.; Atyabi, F.; Vahabpour, R. Optimization of chitosan nanoparticles as an anti-HIV siRNA delivery vehicle. Int. J. Biol. Macromol. 2019, 129, 305–315. [Google Scholar] [CrossRef]
- Patel, P.; Agrawal, Y.K. Targeting nanocarriers containing antisense oligonucleotides to cancer cell. J. Drug Deliv. Sci. Technol. 2017, 37, 97–114. [Google Scholar] [CrossRef]
- Shukla, S.; Sumaria, C.S.; Pradeepkumar, P.I. Exploring chemical modifications for siRNA therapeutics: A structural and functional outlook. ChemMedChem 2010, 5, 328–349. [Google Scholar] [CrossRef]
- Hong, C.A.; Nam, Y.S. Functional Nanostructures for Effective Delivery of Small Interfering RNA Therapeutics. Theranostics 2014, 4, 1211–1232. [Google Scholar] [CrossRef]
- Mao, S.; Sun, W.; Kissel, T. Chitosan-based formulations for delivery of DNA and siRNA. Adv. Drug Deliv. Rev. 2010, 62, 12–27. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Choi, Y.J.; Park, I.K.; Akaike, T.; Cho, C.S. Chemical modification of chitosan with pH-sensitive molecules and specific ligands for efficient DNA transfection and siRNA silencing. J. Nanosci. Nanotechnol. 2014, 14, 564–576. [Google Scholar] [CrossRef] [PubMed]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Adesina, S.K.; Akala, E.O. Nanotechnology Approaches for the Delivery of Exogenous siRNA for HIV Therapy. Mol. Pharm. 2015, 12, 4175–4187. [Google Scholar] [CrossRef] [PubMed]
- Tekade, R.K.; Tekade, M.; Kesharwani, P.; D’Emanuele, A. RNAi-combined nano-chemotherapeutics to tackle resistant tumors. Drug Discov. Today 2016, 21, 1761–1774. [Google Scholar] [CrossRef] [PubMed]
- Weblet Importer. Available online: https://www.sec.gov/Archives/edgar/data/1178670/000115752319000264/a51937655ex99_1.htm (accessed on 19 March 2019).
- FDA approves first-of-its kind targeted RNA-based therapy to treat a rare disease. Available online: https://www.fda.gov/news-events/press-announcements/fda-approves-first-its-kind-targeted-rna-based-therapy-treat-rare-disease (accessed on 11 July 2019).
- Petrilli, R.; Eloy, J.O.; de Souza, M.C.; Abriata Barcellos, J.P.; Marchetti, J.M.; Yung, B.; Lee, R.J. Lipid nanoparticles as non-viral vectors for siRNA delivery: Concepts and applications. In Nanobiomaterials in Drug Delivery: Applications of Nanobiomaterials; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 75–109. ISBN 9780323428897. [Google Scholar]
- A Phase II Study of siG12D LODER in Combination with Chemotherapy in Patients with Unresectable Locally Advanced Pancreatic Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT01676259 (accessed on 17 May 2019).
- Kaczmarek, J.C.; Kowalski, P.S.; Anderson, D.G. Advances in the delivery of RNA therapeutics: From concept to clinical reality. Genome Med. 2017, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Joye, I.J.; McClements, D.J. Biopolymer-based nanoparticles and microparticles: Fabrication, characterization, and application. Curr. Opin. Colloid Interface Sci. 2014, 19, 417–427. [Google Scholar] [CrossRef]
- Raemdonck, K.; Martens, T.F.; Braeckmans, K.; Demeester, J.; De Smedt, S.C. Polysaccharide-based nucleic acid nanoformulations. Adv. Drug Deliv. Rev. 2013, 65, 1123–1147. [Google Scholar] [CrossRef]
- Mokhtarzadeh, A.; Alibakhshi, A.; Hejazi, M.; Omidi, Y.; Dolatabadi, J.E.N. Bacterial-derived biopolymers: Advanced natural nanomaterials for drug delivery and tissue engineering. Trends Anal. Chem. 2016, 82, 367–384. [Google Scholar] [CrossRef]
- Khan, W.; Hosseinkhani, H.; Ickowicz, D.; Hong, P.D.; Yu, D.S.; Domb, A.J. Polysaccharide gene transfection agents. Acta Biomater. 2012, 8, 4224–4232. [Google Scholar] [CrossRef]
- Karunaratne, D.N.; Jafari, M.; Ranatunga, R.J.K.U.; Siriwardhana, A. Natural Carriers for siRNA Delivery. Curr. Pharm. Des. 2015, 21, 4529–4540. [Google Scholar] [CrossRef] [PubMed]
- Myrick, J.M.; Vendra, V.K.; Krishnan, S. Self-assembled polysaccharide nanostructures for controlled-release applications. Nanotechnol. Rev. 2014, 3, 319–346. [Google Scholar] [CrossRef]
- Parraga, J.E.; Zorzi, G.K.; Diebold, Y.; Seijo, B.; Sanchez, A. Nanoparticles based on naturally-occurring biopolymers as versatile delivery platforms for delicate bioactive molecules: An application for ocular gene silencing. Int. J. Pharm. 2014, 477, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Hess, M.; Jones, R.G.; Kahovec, J.; Kitayama, T.; Kratochvíl, P.; Kubisa, P.; Mormann, W.; Stepto, R.F.T.; Tabak, D.; Vohlídal, J.; et al. Terminology of polymers containing ionizable or ionic groups and of polymers containing ions (IUPAC recommendations 2006). Pure Appl. Chem. 2006, 78, 2067–2074. [Google Scholar] [CrossRef]
- Marín-Menéndez, A.; Montis, C.; Díaz-Calvo, T.; Carta, D.; Hatzixanthis, K.; Morris, C.J.; McArthur, M.; Berti, D. Antimicrobial Nanoplexes meet Model Bacterial Membranes: The key role of Cardiolipin. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Svarovsky, S.A.; Gonzalez-Moa, M.J.; Robida, M.D.; Borovkov, A.Y.; Sykes, K. Self-Assembled Micronanoplexes for Improved Biolistic Delivery of Nucleic Acids. Mol. Pharm. 2009, 6, 1927–1933. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Penet, M.F.; Wildes, F.; Takagi, T.; Chen, Z.; Winnard, P.T.; Artemov, D.; Bhujwalla, Z.M. Nanoplex delivery of siRNA and prodrug enzyme for multimodality image-guided molecular pathway targeted cancer therapy. ACS Nano 2010, 4, 6707–6716. [Google Scholar] [CrossRef]
- Buschmann, M.D.; Merzouki, A.; Lavertu, M.; Thibault, M.; Jean, M.; Darras, V. Chitosans for delivery of nucleic acids. Adv. Drug Deliv. Rev. 2013, 65, 1234–1270. [Google Scholar] [CrossRef]
- Chen, W.; Li, H.; Liu, Z.; Yuan, W. Lipopolyplex for therapeutic gene delivery and its application for the treatment of Parkinson’s disease. Front. Aging Neurosci. 2016, 8, 68. [Google Scholar] [CrossRef]
- Insua, I.; Wilkinson, A.; Fernandez-Trillo, F. Polyion complex (PIC) particles: Preparation and biomedical applications. Eur. Polym. J. 2016, 81, 198–215. [Google Scholar] [CrossRef] [Green Version]
- Debele, T.A.; Mekuria, S.L.; Tsai, H.C. Polysaccharide based nanogels in the drug delivery system: Application as the carrier of pharmaceutical agents. Mater. Sci. Eng. C 2016, 68, 964–981. [Google Scholar] [CrossRef]
- Wang, H.; Qian, J.; Ding, F. Recent advances in engineered chitosan-based nanogels for biomedical applications. J. Mater. Chem. B 2017, 5, 6986–7007. [Google Scholar] [CrossRef]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Vauthier, C.; Zandanel, C.; Ramon, A.L. Chitosan-based nanoparticles for in vivo delivery of interfering agents including siRNA. Curr. Opin. Colloid Interface Sci. 2013, 18, 406–418. [Google Scholar] [CrossRef]
- Zhang, N.; Wardwell, P.R.; Bader, R.A. Polysaccharide-Based Micelles for Drug Delivery. Pharmaceutics 2013, 5, 329–352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amjad, M.W.; Kesharwani, P.; Mohd Amin, M.C.I.; Iyer, A.K. Recent advances in the design, development, and targeting mechanisms of polymeric micelles for delivery of siRNA in cancer therapy. Prog. Polym. Sci. 2017, 64, 154–181. [Google Scholar] [CrossRef]
- Ding, F.; Deng, H.; Du, Y.; Shi, X.; Wang, Q. Nanoscale Emerging chitin and chitosan nano fi brous materials for biomedical applications. Nanoscale 2014, 6, 9477–9493. [Google Scholar] [CrossRef]
- Ivanova, E.P.; Bazaka, K.; Crawford, R.J. Natural polymer biomaterials: Advanced applications. New Funct. Biomater. Med. Healthc. 2014, 1, 32–70. [Google Scholar]
- Ragelle, H.; Vandermeulen, G.; Préat, V. Chitosan-based siRNA delivery systems. J. Control. Release 2013, 172, 207–218. [Google Scholar] [CrossRef]
- Mohammed, M.; Syeda, J.; Wasan, K.; Wasan, E. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef]
- Sonaje, K.; Lin, K.J.; Tseng, M.T.; Wey, S.P.; Su, F.Y.; Chuang, E.Y.; Hsu, C.W.; Chen, C.T.; Sung, H.W. Effects of chitosan-nanoparticle-mediated tight junction opening on the oral absorption of endotoxins. Biomaterials 2011, 32, 8712–8721. [Google Scholar] [CrossRef] [PubMed]
- Ranaldi, G.; Marigliano, I.; Vespignani, I.; Perozzi, G.; Sambuy, Y. The effect of chitosan and other polycations on tight junction permeability in the human intestinal Caco-2 cell line. J. Nutr. Biochem. 2002, 13, 157–167. [Google Scholar] [CrossRef]
- Dodane, V.; Amin Khan, M.; Merwin, J.R. Effect of chitosan on epithelial permeability and structure. Int. J. Pharm. 1999, 182, 21–32. [Google Scholar] [CrossRef]
- Rodrigues, S.; Dionísio, M.; López, C.R.; Grenha, A. Biocompatibility of Chitosan Carriers with Application in Drug Delivery. J. Funct. Biomater. 2012, 3, 615–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Howard, K.A.; Dong, M.; Andersen, M.; Rahbek, U.L.; Johnsen, M.G.; Hansen, O.C.; Besenbacher, F.; Kjems, J. The influence of polymeric properties on chitosan/siRNA nanoparticle formulation and gene silencing. Biomaterials 2007, 28, 1280–1288. [Google Scholar] [CrossRef]
- Malmo, J.; Sørgård, H.; Vårum, K.M.; Strand, S.P. SiRNA delivery with chitosan nanoparticles: Molecular properties favoring efficient gene silencing. J. Control. Release 2012, 158, 261–268. [Google Scholar] [CrossRef]
- Holzerny, P.; Ajdini, B.; Heusermann, W.; Bruno, K.; Schuleit, M.; Meinel, L.; Keller, M. Biophysical properties of chitosan/siRNA polyplexes: Profiling the polymer/siRNA interactions and bioactivity. J. Control. Release 2012, 157, 297–304. [Google Scholar] [CrossRef]
- Katas, H.; Alpar, H.O. Development and characterisation of chitosan nanoparticles for siRNA delivery. J. Control. Release 2006, 115, 216–225. [Google Scholar] [CrossRef]
- Raja, M.A.G.; Katas, H.; Wen, T.J. Stability, intracellular delivery, and release of siRNA from chitosan nanoparticles using different cross-linkers. PLoS ONE 2015, 10, 1–19. [Google Scholar]
- Jean, M.; Alameh, M.; De Jesus, D.; Thibault, M.; Lavertu, M.; Darras, V.; Nelea, M.; Buschmann, M.D.; Merzouki, A. Chitosan-based therapeutic nanoparticles for combination gene therapy and gene silencing of in vitro cell lines relevant to type 2 diabetes. Eur. J. Pharm. Sci. 2012, 45, 138–149. [Google Scholar] [CrossRef]
- Alameh, M.; DeJesus, D.; Jean, M.; Darras, V.; Thibault, M.; Lavertu, M.; Buschmann, M.D.; Merzouki, A. Low molecular weight chitosan nanoparticulate system at low N:P ratio for nontoxic polynucleotide delivery. Int. J. Nanomed. 2012, 7, 1399–1414. [Google Scholar] [Green Version]
- Ballarín-González, B.; Dagnaes-Hansen, F.; Fenton, R.A.; Gao, S.; Hein, S.; Dong, M.; Kjems, J.; Howard, K. a Protection and Systemic Translocation of siRNA Following Oral Administration of Chitosan/siRNA Nanoparticles. Mol. Ther. Nucleic Acids 2013, 2, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Farid, M.M.; Hathout, R.M.; Fawzy, M.; Abou-Aisha, K. Silencing of the scavenger receptor (Class B—Type 1) gene using siRNA-loaded chitosan nanaoparticles in a HepG2 cell model. Colloids Surf. B Biointerfaces 2014, 123, 930–937. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, X.; Zhang, D.; Yu, W.; Lv, G.; Xie, H.; Zheng, J.; Ma, X. Chitosan/VEGF-sIRNA nanoparticle for gene silencing. J. Control. Release 2011, 152, e160–e161. [Google Scholar] [CrossRef] [PubMed]
- Huth, S.; Schmidt, C.; Rudolph, C.; Rosenecker, J. 703. Analysis of the Stability and Functionality of siRNA after Nebulization of siRNA Polyplexes. Mol. Ther. 2006, 13, S272. [Google Scholar] [CrossRef]
- Sharma, K.; Somavarapu, S.; Colombani, A.; Govind, N.; Taylor, K.M.G. Nebulised siRNA encapsulated crosslinked chitosan nanoparticles for pulmonary delivery. Int. J. Pharm. 2013, 455, 241–247. [Google Scholar] [CrossRef] [PubMed]
- Mittnacht, U.; Hartmann, H.; Hein, S.; Oliveira, H.; Dong, M.; Pêgo, A.P.; Kjems, J.; Howard, K.A.; Schlosshauer, B. Chitosan/siRNA nanoparticles biofunctionalize nerve implants and enable neurite outgrowth. Nano Lett. 2010, 10, 3933–3939. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, N.; Mittnacht, U.; Hartmann, H.; Baumer, Y.; Kjems, J.; Oberhoffner, S.; Schlosshauer, B. Neuronal and glial responses to siRNA-coated nerve guide implants in vitro. Neurosci. Lett. 2011, 494, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, J.; Zhu, K.Y. Chitosan/double-stranded RNA nanoparticle-mediated RNA interference to silence chitin synthase genes through larval feeding in the African malaria mosquito (Anopheles gambiae). Insect Mol. Biol. 2010, 19, 683–693. [Google Scholar] [CrossRef] [PubMed]
- Mysore, K.; Flannery, E.M.; Tomchaney, M.; Severson, D.W.; Duman-Scheel, M. Disruption of Aedes aegypti Olfactory System Development through Chitosan/siRNA Nanoparticle Targeting of semaphorin-1a. PLoS Negl. Trop. Dis. 2013, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Van Woensel, M.; Wauthoz, N.; Rosière, R.; Mathieu, V.; Kiss, R.; Lefranc, F.; Steelant, B.; Dilissen, E.; Van Gool, S.W.; Mathivet, T.; et al. Development of siRNA-loaded chitosan nanoparticles targeting Galectin-1 for the treatment of glioblastoma multiforme via intranasal administration. J. Control. Release 2016, 227, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Nilsson, L.; Cheema, M.U.; Wang, Y.; Frøkiær, J.; Gao, S.; Kjems, J.; Nørregaard, R. Chitosan/siRNA nanoparticles targeting cyclooxygenase type 2 attenuate unilateral ureteral obstruction-induced kidney injury in mice. Theranostics 2015, 5, 110–123. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Bhatt, P.; Lalani, R.; Amrutiya, J.; Vhora, I.; Kolte, A.; Misra, A. Low molecular weight chitosan–protamine conjugate for siRNA delivery with enhanced stability and transfection efficiency. RSC Adv. 2016, 6, 110951–110963. [Google Scholar] [CrossRef]
- Nascimento, A.V.; Gattacceca, F.; Singh, A.; Bousbaa, H.; Ferreira, D.; Sarmento, B.; Amiji, M.M. Biodistribution and pharmacokinetics of Mad2 siRNA-loaded EGFR-targeted chitosan nanoparticles in cisplatin sensitive and resistant lung cancer models. Nanomedicine 2016, 11, 767–781. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, A.V.; Singh, A.; Bousbaa, H.; Ferreira, D.; Sarmento, B.; Amiji, M.M. Overcoming cisplatin resistance in non-small cell lung cancer with Mad2 silencing siRNA delivered systemically using EGFR-targeted chitosan nanoparticles. Acta Biomater. 2017, 47, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Huang, W.; Jin, M.; Wang, Q.; Fan, B.; Kang, L.; Gao, Z. Chitosan-based nanoparticles for survivin targeted siRNA delivery in breast tumor therapy and preventing its metastasis. Int. J. Nanomed. 2016, 11, 4931–4945. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.-G.; Zhu, W.; Liu, Y.; Yuan, Z.; Yang, S.; Chen, W.; Li, J.; Zhou, X.; Liu, C.; Zhang, X. Novel polymer micelle mediated co-delivery of doxorubicin and P-glycoprotein siRNA for reversal of multidrug resistance and synergistic tumor therapy. Sci. Rep. 2016, 6, 23859. [Google Scholar] [CrossRef]
- Corbet, C.; Ragelle, H.; Pourcelle, V.; Vanvarenberg, K.; Marchand-Brynaert, J.; Préat, V.; Feron, O. Delivery of siRNA targeting tumor metabolism using non-covalent PEGylated chitosan nanoparticles: Identification of an optimal combination of ligand structure, linker and grafting method. J. Control. Release 2016, 223, 53–63. [Google Scholar] [CrossRef]
- Yang, S.D.; Zhu, W.J.; Zhu, Q.L.; Chen, W.L.; Ren, Z.X.; Li, F.; Yuan, Z.Q.; Li, J.Z.; Liu, Y.; Zhou, X.F.; et al. Binary-copolymer system base on low-density lipoprotein-coupled N-succinyl chitosan lipoic acid micelles for co-delivery MDR1 siRNA and paclitaxel, enhances antitumor effects via reducing drug. J. Biomed. Mater. Res. Part B Appl. Biomater. 2016, 00B, 1–12. [Google Scholar] [CrossRef]
- He, C.; Yin, L.; Song, Y.; Tang, C.; Yin, C. Optimization of multifunctional chitosan-siRNA nanoparticles for oral delivery applications, targeting TNF-a silencing in rats. Acta Biomater. 2015, 17, 98–106. [Google Scholar] [CrossRef]
- Lee, S.S.J.; Yook, S.; Yhee, J.Y.; Yoon, H.Y.; Kim, M.G.; Ku, S.H.; Kim, S.H.; Park, J.H.; Jeong, J.H.; Kwon, I.C.; et al. Co-delivery of VEGF and Bcl-2 dual-targeted siRNA polymer using a single nanoparticle for synergistic anti-cancer effects in vivo. J. Control. Release 2015, 220, 631–641. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.Y.; Son, S.; Lee, S.S.J.; You, D.G.; Yhee, J.Y.; Park, J.H.; Swierczewska, M.; Lee, S.S.J.; Kwon, I.C.; Kim, S.H.; et al. Glycol chitosan nanoparticles as specialized cancer therapeutic vehicles: Sequential delivery of doxorubicin and Bcl-2 siRNA. Sci. Rep. 2014, 4, 6878. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Huang, W.; Li, Y.; Liu, S.; Jin, M.; Wang, Y.; Jia, L.; Gao, Z. Anti-tumor effects in mice induced by survivin-targeted siRNA delivered through polysaccharide nanoparticles. Biomaterials 2013, 34, 5689–5699. [Google Scholar] [CrossRef] [PubMed]
- Sun, P.; Huang, W.; Kang, L.; Jin, M.; Fan, B.; Jin, H.; Wang, Q.-M.; Gao, Z. Chitosan Nanoparticle with Enhanced Cell- Penetrating and Endosomal Escape Capacities for Suppressing Breast Tumor Metastasis. Int. J. Nanomed. 2017, 12, 3221–3234. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Li, J. Targeted siRNA delivery reduces nitric oxide mediated cell death after spinal cord injury. J. Nanobiotechnol. 2017, 15, 38. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Tang, C.; Yin, C. Combination antitumor immunotherapy with VEGF and PIGF siRNA via systemic delivery of multi-functionalized nanoparticles to tumor-associated macrophages and breast cancer cells. Biomaterials 2018, 185, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Shi, Q.; Rondon-Cavanzo, E.P.; Dalla Picola, I.P.; Tiera, M.J.; Zhang, X.; Dai, K.; Benabdoune, H.A.; Benderdour, M.; Fernandes, J.C. In vivo therapeutic efficacy of TNFα silencing by folate-PEG-chitosan-DEAE/siRNA nanoparticles in arthritic mice. Int. J. Nanomed. 2018, 13, 387–402. [Google Scholar] [CrossRef] [PubMed]
- Sadio, A.; Gustafsson, J.K.; Pereira, B.; Gomes, C.P.; Hansson, G.C.; David, L.; Pêgo, A.P.; Almeida, R. Modified-Chitosan/siRNA Nanoparticles Downregulate Cellular CDX2 Expression and Cross the Gastric Mucus Barrier. PLoS ONE 2014, 9, e99449. [Google Scholar] [CrossRef] [PubMed]
- Sadreddini, S.; Safaralizadeh, R.; Baradaran, B.; Aghebati-Maleki, L.; Hosseinpour-Feizi, M.A.; Shanehbandi, D.; Jadidi-Niaragh, F.; Sadreddini, S.; Kafil, H.S.; Younesi, V.; et al. Chitosan nanoparticles as a dual drug/siRNA delivery system for treatment of colorectal cancer. Immunol. Lett. 2017, 181, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Siahmansouri, H.; Somi, M.H.; Babaloo, Z.; Baradaran, B.; Jadidi-Niaragh, F.; Atyabi, F.; Mohammadi, H.; Ahmadi, M.; Yousefi, M. Effects of HMGA2 siRNA and doxorubicin dual delivery by chitosan nanoparticles on cytotoxicity and gene expression of HT-29 colorectal cancer cell line. J. Pharm. Pharmacol. 2016, 68, 1119–1130. [Google Scholar] [CrossRef] [PubMed]
- Afkham, A.; Aghebati-Maleki, L.; Siahmansouri, H.; Sadreddini, S.; Ahmadi, M.; Dolati, S.; Afkham, N.M.; Akbarzadeh, P.; Jadidi-Niaragh, F.; Younesi, V.; et al. Chitosan (CMD)-mediated co-delivery of SN38 and Snail-specific siRNA as a useful anticancer approach against prostate cancer. Pharmacol. Rep. 2018, 70, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Muddineti, O.S.; Shah, A.; Rompicharla, S.V.K.; Ghosh, B.; Biswas, S. Cholesterol-grafted chitosan micelles as a nanocarrier system for drug-siRNA co-delivery to the lung cancer cells. Int. J. Biol. Macromol. 2018, 118, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Jadidi-Niaragh, F.; Atyabi, F.; Rastegari, A.; Mollarazi, E.; Kiani, M.; Razavi, A.; Yousefi, M.; Kheshtchin, N.; Hassannia, H.; Hadjati, J.; et al. Downregulation of CD73 in 4T1 breast cancer cells through siRNA-loaded chitosan-lactate nanoparticles. Tumor Biol. 2016, 37, 8403–8412. [Google Scholar] [CrossRef] [PubMed]
- Rudzinski, W.E.; Palacios, A.; Ahmed, A.; Lane, M.A.; Aminabhavi, T.M. Targeted delivery of small interfering RNA to colon cancer cells using chitosan and PEGylated chitosan nanoparticles. Carbohydr. Polym. 2016, 147, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.P.; Nah, J.W. Target gene delivery from targeting ligand conjugated chitosan-PEI copolymer for cancer therapy. Carbohydr. Polym. 2016, 135, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Gutoaia, A.; Schuster, L.; Margutti, S.; Laufer, S.; Schlosshauer, B.; Krastev, R.; Stoll, D.; Hartmann, H. Fine-tuned PEGylation of chitosan to maintain optimal siRNA-nanoplex bioactivity. Carbohydr. Polym. 2016, 143, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Ma, P.; Viennois, E.; Merlin, D. Urocanic acid-modified chitosan nanoparticles can confer anti-inflammatory effect by delivering CD98 siRNA to macrophages. Colloids Surf. B Biointerfaces 2016, 143, 186–193. [Google Scholar] [CrossRef] [Green Version]
- Ragelle, H.; Colombo, S.; Pourcelle, V.; Vanvarenberg, K.; Vandermeulen, G.; Bouzin, C.; Marchand-Brynaert, J.; Feron, O.; Foged, C.; Préat, V. Intracellular siRNA delivery dynamics of integrin-targeted, PEGylated chitosan–poly(ethylene imine) hybrid nanoparticles: A mechanistic insight. J. Control. Release 2015, 211, 1–9. [Google Scholar] [CrossRef]
- Ragelle, H.; Riva, R.; Vandermeulen, G.; Naeye, B.; Pourcelle, V.; Le Duff, C.S.; D’Haese, C.; Nysten, B.; Braeckmans, K.; De Smedt, S.C.; et al. Chitosan nanoparticles for siRNA delivery: Optimizing formulation to increase stability and efficiency. J. Control. Release 2014, 176, 54–63. [Google Scholar] [CrossRef]
- He, C.; Yin, L.; Tang, C.; Yin, C. Multifunctional polymeric nanoparticles for oral delivery of TNF-α siRNA to macrophages. Biomaterials 2013, 34, 2843–2854. [Google Scholar] [CrossRef]
- Malhotra, M.; Tomaro-Duchesneau, C.; Prakash, S. Synthesis of TAT peptide-tagged PEGylated chitosan nanoparticles for siRNA delivery targeting neurodegenerative diseases. Biomaterials 2013, 34, 1270–1280. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Jeong, E.J.; Lee, J.; Rhim, T.; Lee, S.K.; Lee, K.Y. Preparation and characterization of nonaarginine-modified chitosan nanoparticles for siRNA delivery. Carbohydr. Polym. 2013, 92, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Sercombe, L.; Veerati, T.; Moheimani, F.; Wu, S.Y.; Sood, A.K.; Hua, S. Advances and Challenges of Liposome Assisted Drug Delivery. Front. Pharmacol. 2015, 6, 286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Matteis, L.; Alleva, M.; Serrano-Sevilla, I.; García-Embid, S.; Stepien, G.; Moros, M.; de la Fuente, J. Controlling Properties and Cytotoxicity of Chitosan Nanocapsules by Chemical Grafting. Mar. Drugs 2016, 14, 175. [Google Scholar] [CrossRef] [PubMed]
- Nag, M.; Gajbhiye, V.; Kesharwani, P.; Jain, N.K. Transferrin functionalized chitosan-PEG nanoparticles for targeted delivery of paclitaxel to cancer cells. Colloids Surf. B Biointerfaces 2016, 148, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Papadimitriou, S.A.; Achilias, D.S.; Bikiaris, D.N. Chitosan-g-PEG nanoparticles ionically crosslinked with poly(glutamic acid) and tripolyphosphate as protein delivery systems. Int. J. Pharm. 2012, 430, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Casettari, L.; Vllasaliu, D.; Mantovani, G.; Howdle, S.M.; Stolnik, S.; Illum, L. Effect of PEGylation on the toxicity and permeability enhancement of chitosan. Biomacromolecules 2010, 11, 2854–2865. [Google Scholar] [CrossRef]
- Moros, M.; Mitchell, S.G.; Grazúa, V.; De La Fuente, J.M. The fate of nanocarriers as nanomedicines in vivo: Important considerations and biological barriers to overcome. Curr. Med. Chem. 2013, 20, 2759–2778. [Google Scholar] [CrossRef]
- Bashir, S.; Teo, Y.Y.; Ramesh, S.; Ramesh, K.; Khan, A.A. N-succinyl chitosan preparation, characterization, properties and biomedical applications: A state of the art review. Rev. Chem. Eng. 2015, 31, 563–597. [Google Scholar] [CrossRef]
- Anitha, A.; Maya, S.; Deepa, N.; Chennazhi, K.P.; Nair, S.V.; Tamura, H.; Jayakumar, R. Efficient water soluble O-carboxymethyl chitosan nanocarrier for the delivery of curcumin to cancer cells. Carbohydr. Polym. 2011, 83, 452–461. [Google Scholar] [CrossRef]
- Eivazy, P.; Atyabi, F.; Jadidi-Niaragh, F.; Aghebati Maleki, L.; Miahipour, A.; Abdolalizadeh, J.; Yousefi, M. The impact of the codelivery of drug-siRNA by trimethyl chitosan nanoparticles on the efficacy of chemotherapy for metastatic breast cancer cell line (MDA-MB-231). Artif. Cells Nanomed. Biotechnol. 2016, 1401, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Xiao, B.; Ma, P.; Ma, L.; Chen, Q.; Si, X.; Walter, L.; Merlin, D. Effects of tripolyphosphate on cellular uptake and RNA interference efficiency of chitosan-based nanoparticles in Raw 264.7 macrophages. J. Colloid Interface Sci. 2017, 490, 520–528. [Google Scholar] [CrossRef] [PubMed]
- Zahir-Jouzdani, F.; Mahbod, M.; Soleimani, M.; Vakhshiteh, F.; Arefian, E.; Shahosseini, S.; Dinarvand, R.; Atyabi, F. Chitosan and thiolated chitosan: Novel therapeutic approach for preventing corneal haze after chemical injuries. Carbohydr. Polym. 2018, 179, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, S.; Serpooshan, V.; Tao, W.; Hamaly, M.A.; Alkawareek, M.Y.; Dreaden, E.C.; Brown, D.; Alkilany, A.M.; Farokhzad, O.C.; Mahmoudi, M. Cellular uptake of nanoparticles: Journey inside the cell. Chem. Soc. Rev. 2017, 46, 4218–4244. [Google Scholar] [CrossRef]
- Liang, W.; Lam, J.K.W. Endosomal Escape Pathways for Non-Viral Nucleic Acid Delivery Systems. In Molecular Regulation of Endocytosis; InTech: London, UK, 2012; pp. 429–456. [Google Scholar]
- Howard, K.A.; Rahbek, U.L.; Liu, X.; Damgaard, C.K.; Glud, S.Z.; Andersen, M.; Hovgaard, M.B.; Schmitz, A.; Nyengaard, J.R.; Besenbacher, F.; et al. RNA Interference in Vitro and in Vivo Using a Novel Chitosan/siRNA Nanoparticle System. Mol. Ther. 2006, 14, 476–484. [Google Scholar] [CrossRef]
- Moreira, C.; Oliveira, H.; Pires, L.R.; Simões, S.; Barbosa, M.A.; Pêgo, A.P. Improving chitosan-mediated gene transfer by the introduction of intracellular buffering moieties into the chitosan backbone. Acta Biomater. 2009, 5, 2995–3006. [Google Scholar] [CrossRef]
- Yang, F.; Li, Y.; Huang, W.; Chen, W.; Jin, M.; Gao, Z. Cell-penetrating peptide TAT modified chitosan for siRNA delivery. J. Control. Release 2013, 172, e100–e101. [Google Scholar] [CrossRef]
- Ni, S.; Liu, Y.; Tang, Y.; Chen, J.; Li, S.; Pu, J.; Han, L. GABABreceptor ligand-directed trimethyl chitosan/tripolyphosphate nanoparticles and their pMDI formulation for survivin siRNA pulmonary delivery. Carbohydr. Polym. 2018, 179, 135–144. [Google Scholar] [CrossRef]
- Gu, J.; Al-Bayati, K.; Ho, E.A. Development of antibody-modified chitosan nanoparticles for the targeted delivery of siRNA across the blood-brain barrier as a strategy for inhibiting HIV replication in astrocytes. Drug Deliv. Transl. Res. 2017, 7, 497–506. [Google Scholar] [CrossRef]
- Boeriu, C.G.; Springer, J.; Kooy, F.K.; van den Broek, L.A.M.; Eggink, G. Production Methods for Hyaluronan. Int. J. Carbohydr. Chem. 2013, 2013, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Kim, K.S.; Jiang, G.; Kang, H.; Kim, S.; Kim, B.S.; Park, M.H.; Hahn, S.K. In vivo real-time bioimaging of hyaluronic acid derivatives using quantum dots. Biopolymers 2008, 89, 1144–1153. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Liang, J.; Noble, P.; Jiang, D.; Noble, P. Hyaluronan as a therapeutic target in human diseases. Physiol. Rev. 2011, 91, 221–264. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.J.; Park, K.; Kim, K.S.; Kim, J.; Yang, J.A.; Kong, J.H.; Lee, M.Y.; Hoffman, A.S.; Hahn, S.K. Target specific and long-acting delivery of protein, peptide, and nucleotide therapeutics using hyaluronic acid derivatives. J. Control. Release 2010, 141, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Lucas, R.; Gómez-Pinto, I.; Aviñó, A.; Reina, J.J.; Eritja, R.; González, C.; Morales, J.C. Highly Polar Carbohydrates Stack onto DNA Duplexes via CH/π Interactions. J. Am. Chem. Soc. 2011, 133, 1909–1916. [Google Scholar] [CrossRef] [PubMed]
- Paidikondala, M.; Rangasami, V.K.; Nawale, G.N.; Casalini, T.; Perale, G.; Kadekar, S.; Mohanty, G.; Salminen, T.; Oommen, O.P.; Varghese, O.P. An Unexpected Role of Hyaluronic Acid in Trafficking siRNA Across the Cellular Barrier: The First Biomimetic, Anionic, Non-Viral Transfection Method. Angew. Chem. Int. Ed. 2019, 58, 2815–2819. [Google Scholar] [CrossRef] [PubMed]
- Forti, E.; Kryukov, O.; Elovic, E.; Goldshtein, M.; Korin, E.; Margolis, G.; Felder, S.; Ruvinov, E.; Cohen, S. A bridge to silencing: Co-assembling anionic nanoparticles of siRNA and hyaluronan sulfate via calcium ion bridges. J. Control. Release 2016, 232, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Mok, H.; Lee, S.; Oh, Y.-K.; Park, T.G. Target-specific intracellular delivery of siRNA using degradable hyaluronic acid nanogels. J. Control. Release 2007, 119, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.M.; Jang, M.; Kim, J.H.; Ahn, H.J. Tumor-specific delivery of siRNA using supramolecular assembly of hyaluronic acid nanoparticles and 2b RNA-binding protein/siRNA complexes. Biomaterials 2014, 35, 7121–7132. [Google Scholar] [CrossRef]
- Al-Qadi, S.; Alatorre-Meda, M.; Zaghloul, E.M.; Taboada, P.; Remunán-López, C. Chitosan-hyaluronic acid nanoparticles for gene silencing: The role of hyaluronic acid on the nanoparticles’ formation and activity. Colloids Surf. B Biointerfaces 2013, 103, 615–623. [Google Scholar] [CrossRef]
- Fukushige, K.; Tagami, T.; Ozeki, T. The offset effect of a hyaluronic acid coating to cationic carriers containing siRNA: Alleviated cytotoxicity and retained gene silencing in vitro. J. Drug Deliv. Sci. Technol. 2017, 39, 435–441. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Wang, B.; Shen, Y.; Ouahab, A. Co-delivery of siRNA and hypericin into cancer cells by hyaluronic acid modified PLGA-PEI nanoparticles. Drug Dev. Ind. Pharm. 2016, 42, 737–746. [Google Scholar] [CrossRef]
- Kosovrasti, V.Y.; Nechev, L.V.; Amiji, M.M. Peritoneal Macrophage-Specific TNF-α Gene Silencing in LPS-Induced Acute Inflammation Model Using CD44 Targeting Hyaluronic Acid Nanoparticles. Mol. Pharm. 2016, 13, 3404–3416. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Ryoo, N.K.; Han, H.; Hong, H.K.; Park, J.Y.; Park, S.J.; Kim, Y.K.; Sim, C.; Kim, K.; Woo, S.J.; et al. Anti-VEGF PolysiRNA Polyplex for the Treatment of Choroidal Neovascularization. Mol. Pharm. 2016, 13, 1988–1995. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.; Zhang, F.; Wei, T.; Zuo, T.; Guan, Y.; Lin, G.; Shao, W. Smart linkers in polymer-drug conjugates for tumor-targeted delivery. J. Drug Target. 2016, 24, 475–491. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Yang, J.-A.; Lee, M.-Y.; Lee, H.; Hahn, S.K. Reducible Hyaluronic Acid–siRNA Conjugate for Target Specific Gene Silencing. Bioconj. Chem. 2013, 24, 1201–1209. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.J.; Zhao, Z.X.; Wang, J.C.; Zhao, E.Y.; Gao, L.Y.; Zhou, S.F.; Liu, X.Y.; Lu, W.L.; Zhang, Q. A comparative study of three ternary complexes prepared in different mixing orders of siRNA/redox-responsive hyperbranched poly (amido amine)/hyaluronic acid. Int. J. Nanomed. 2012, 7, 3837–3849. [Google Scholar] [Green Version]
- Yin, T.; Liu, J.; Zhao, Z.; Dong, L.; Cai, H.; Yin, L.; Zhou, J.; Huo, M. Smart nanoparticles with a detachable outer shell for maximized synergistic antitumor efficacy of therapeutics with varying physicochemical properties. J. Control. Release 2016, 243, 54–68. [Google Scholar] [CrossRef]
- Shen, Y.; Wang, J.; Li, Y.; Tian, Y.; Sun, H.; Ammar, O.; Tu, J.; Wang, B.; Sun, C. Co-delivery of siRNA and paclitaxel into cancer cells by hyaluronic acid modified redox-sensitive disulfide-crosslinked PLGA–PEI nanoparticles. RSC Adv. 2015, 5, 46464–46479. [Google Scholar] [CrossRef]
- Hyun, E.; Hasan, M.N.; Kang, S.H.; Cho, S.; Lee, Y. Oral siRNA delivery using dual transporting systems to efficiently treat colorectal liver metastasis. Int. J. Pharm. 2019, 555, 250–258. [Google Scholar] [CrossRef]
- Ding, J.; Liang, T.; Min, Q.; Jiang, L.; Zhu, J.J. “Stealth and Fully-Laden” Drug Carriers: Self-Assembled Nanogels Encapsulated with Epigallocatechin Gallate and siRNA for Drug-Resistant Breast Cancer Therapy. ACS Appl. Mater. Interfaces 2018, 10, 9938–9948. [Google Scholar] [CrossRef]
- Yin, T.; Wang, L.; Yin, L.; Zhou, J.; Huo, M. Co-delivery of hydrophobic paclitaxel and hydrophilic AURKA specific siRNA by redox-sensitive micelles for effective treatment of breast cancer. Biomaterials 2015, 61, 10–25. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, S.; Iyer, A.K.; Morrissey, D.V.; Amiji, M.M. Hyaluronic acid based self-assembling nanosystems for CD44 target mediated siRNA delivery to solid tumors. Biomaterials 2013, 34, 3489–3502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganesh, S.; Iyer, A.K.; Gattacceca, F.; Morrissey, D.V.; Amiji, M.M. In vivo biodistribution of siRNA and cisplatin administered using CD44-targeted hyaluronic acid nanoparticles. J. Control. Release 2013, 172, 699–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganesh, S.; Iyer, A.K.; Weiler, J.; Morrissey, D.V.; Amiji, M.M. Combination of siRNA-directed Gene Silencing with Cisplatin Reverses Drug Resistance in Human Non-small Cell Lung Cancer. Mol. Ther. Nucleic Acids 2013, 2, e110. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhang, M.; Liu, Y.; Li, C.; Zhang, Q.; Oupicky, D.; Sun, M. Reversible Covalent Cross-Linked Polycations with Enhanced Stability and ATP-Responsive Behavior for Improved siRNA Delivery. Biomacromolecules 2018, 19, 3776–3787. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Gao, Y.; Wu, Y.; An, C. The AIB1siRNA-loaded hyaluronic acid-assembled PEI/heparin/Ca2+ nanocomplex as a novel therapeutic strategy in lung cancer treatment. Int. J. Mol. Med. 2019, 43, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Wang, B.; Lu, Y.; Ouahab, A.; Li, Q.; Tu, J. A novel tumor-targeted delivery system with hydrophobized hyaluronic acid-spermine conjugates (HHSCs) for efficient receptor-mediated siRNA delivery. Int. J. Pharm. 2011, 414, 233–243. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.Y.; Kim, H.R.; Saravanakumar, G.; Heo, R.; Chae, S.Y.; Um, W.; Kim, K.; Kwon, I.C.; Lee, J.Y.; Lee, D.S.; et al. Bioreducible hyaluronic acid conjugates as siRNA carrier for tumor targeting. J. Control. Release 2013, 172, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Parmar, M.B.; Meenakshi Sundaram, D.N.; Bahadur, K.C.R.; Maranchuk, R.; Montazeri Aliabadi, H.; Hugh, J.C.; Löbenberg, R.; Uludağ, H. Combinational siRNA delivery using hyaluronic acid modified amphiphilic polyplexes against cell cycle and phosphatase proteins to inhibit growth and migration of triple-negative breast cancer cells. Acta Biomater. 2018, 66, 294–309. [Google Scholar] [CrossRef] [Green Version]
- De Koker, S.; Hoogenboom, R.; De Geest, B.G. Polymeric multilayer capsules for drug delivery. Chem. Soc. Rev. 2012, 41, 2867. [Google Scholar] [CrossRef]
- Kim, E.-J.; Shim, G.; Kim, K.; Kwon, I.C.; Oh, Y.-K.; Shim, C.-K. Hyaluronic acid complexed to biodegradable poly L-arginine for targeted delivery of siRNAs. J. Gene Med. 2009, 11, 791–803. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, S.K.; Gupta, S.; Gupta, K.C.; Kumar, P. Efficient DNA and siRNA delivery with biodegradable cationic hyaluronic acid conjugates. RSC Adv. 2013, 3, 15687. [Google Scholar] [CrossRef]
- Aruffo, A.; Stamenkovic, I.; Melnick, M.; Underhill, C.B.; Seed, B. CD44 is the principle cell surface receptor for hyaluronate. Cell 1990, 61, 1303–1313. [Google Scholar] [CrossRef]
- Entwistle, J.; Turley, E.A.; Underhill, C.D. HA Receptors: Regulators of Signalling to the Cytoskeleton. J. Cell. Biochem. 1996, 61, 569–577. [Google Scholar] [CrossRef]
- Zhou, B.; Weigel, J.A.; Fauss, L.; Weigel, P.H. Identification of the hyaluronan receptor for endocytosis (HARE). J. Biol. Chem. 2000, 275, 37733–37741. [Google Scholar] [CrossRef]
- Yoon, S.K.; Hong, S.W.; Sung, P.S.; Park, C.; Song, M.J.; Yang, J.M.; Choi, S.W.; Lee, C.D.; Lee, Y.S.; Hahn, S.K. Targeted Therapy for Liver Cirrhosis Using Hyaluronic Acid (Ha) Conjugated TgfB1 Sirna. J. Hepatol. 2011, 54, S422. [Google Scholar] [CrossRef]
- Lin, L.; Cai, M.; Deng, S.; Huang, W.; Huang, J.; Huang, X.; Huang, M.; Wang, Y.; Shuai, X.; Zhu, K. Amelioration of cirrhotic portal hypertension by targeted cyclooxygenase-1 siRNA delivery to liver sinusoidal endothelium with polyethylenimine grafted hyaluronic acid. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2329–2339. [Google Scholar] [CrossRef]
- Choi, K.Y.K.; Min, K.H.; Yoon, H.Y.; Kim, K.; Park, J.H.; Kwon, I.C.; Choi, K.Y.K.; Jeong, S.Y. PEGylation of hyaluronic acid nanoparticles improves tumor targetability in vivo. Biomaterials 2011, 32, 1880–1889. [Google Scholar] [CrossRef]
- Mizrahy, S.; Raz, S.R.; Hasgaard, M.; Liu, H.; Soffer-Tsur, N.; Cohen, K.; Dvash, R.; Landsman-Milo, D.; Bremer, M.G.E.G.; Moghimi, S.M.; et al. Hyaluronan-coated nanoparticles: The influence of the molecular weight on CD44-hyaluronan interactions and on the immune response. J. Control. Release 2011, 156, 231–238. [Google Scholar] [CrossRef]
- Byeon, Y.; Lee, J.-W.; Choi, W.S.; Won, J.E.; Kim, G.H.; Kim, M.G.; Wi, T.I.; Lee, J.M.; Kang, T.H.; Jung, I.D.; et al. CD44-targeted PLGA nanoparticles incorporating paclitaxel and FAK siRNA overcome chemoresistance in epithelial ovarian cancer. Cancer Res. 2018, 78, 6247–6256. [Google Scholar] [CrossRef] [Green Version]
- Yang, X.; Iyer, A.K.; Singh, A.; Choy, E.; Hornicek, F.J.; Amiji, M.M.; Duan, Z. MDR1 siRNA loaded hyaluronic acid-based CD44 targeted nanoparticle systems circumvent paclitaxel resistance in ovarian cancer. Sci. Rep. 2015, 5, 8509. [Google Scholar] [CrossRef]
- Tirella, A.; Kloc-Muniak, K.; Good, L.; Ridden, J.; Ashford, M.; Puri, S.; Tirelli, N. CD44 targeted delivery of siRNA by using HA-decorated nanotechnologies for KRAS silencing in cancer treatment. Int. J. Pharm. 2019, 561, 114–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, G.H.; Won, J.E.; Byeon, Y.; Kim, M.G.; Wi, T.I.; Lee, J.M.; Park, Y.Y.; Lee, J.W.; Kang, T.H.; Jung, I.D.; et al. Selective delivery of PLXDC1 small interfering RNA to endothelial cells for anti-angiogenesis tumor therapy using CD44-targeted chitosan nanoparticles for epithelial ovarian cancer. Drug Deliv. 2018, 25, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Veilleux, D.; Gopalakrishna Panicker, R.K.; Chevrier, A.; Biniecki, K.; Lavertu, M.; Buschmann, M.D. Lyophilisation and concentration of chitosan/siRNA polyplexes: Influence of buffer composition, oligonucleotide sequence, and hyaluronic acid coating. J. Colloid Interface Sci. 2018, 512, 335–345. [Google Scholar] [CrossRef] [PubMed]









| Components of the Delivery System | CS Modifications | Improvement of the Modification | In vitro/In vivo Study | Combination Therapy | Ref |
|---|---|---|---|---|---|
| Delivery system 1. Non-target delivery: CS, PEG, siRNA Delivery system 2. Target delivery: CS, PEG, EGFR-targeting peptide, siRNA | PEG EGFR peptide | Colloidal stability and longer half-life Target EGF receptor (EGFR) | In vitro + in vivo | Sequential delivery: 1. NPs siRNA 2. cisplatin | [78,79] |
| CS, PEG, siRNA | PEG | Solubility and stability | In vitro + in vivo | - | [80] |
| N-succinyl-CS, doxorubicin, poly-l-lysine (PLL), palmitic acid, siRNA | Succinyl PLL Palmitic acid | Solubility siRNA binding Hydrophobic core | In vitro + in vivo | Co-delivery: siRNA doxorubicin | [81] |
| CS, PEI, TPP, PEG, RGDp, siRNA | PEG RGDp | Solubility and stability Target αvβ3 integrin receptors | In vitro + in vivo | Co-delivery: 2 siRNAs | [82] |
| N-succinyl CS, paclitaxel, lipoic acid (LA), low-density lipoprotein (LDL), cholesterol-siRNA | Succinyl LA LDL Cholesterol | Solubility Hydrophobic core Target LDL receptor Hydrophobic interactions with LA | In vitro + in vivo | Co-delivery: siRNA paclitaxel | [83] |
| Low Molecular weight (LMw) CS, protamine, TPP, siRNA | LMw CS Protamine | Solubility and colloidal stability siRNA binding and cell uptake | In vitro + in vivo | - | [77] |
| Trimethyl-CS, cysteine, mannose, siRNA | Trimethyl Cysteine Mannose | Solubility Stability (disulphide bonds) Target enterocites and M cells (intestinal absorption) and macrophages | In vitro + in vivo | - | [84] |
| Glycol-CS, sulfosuccinimidyl 6-[3′(2-pyridyldithio)-propionamido] hexanoate (Sulfo-LC-SPDP), dual-poly-siRNA | Glycol Sulfo-LC-SPDP | Solubility Stability (disulphide bonds) | In vitro + in vivo | Co-delivery: 2 siRNAs | [85] |
| Delivery system 1. siRNA-loaded: Glycol-CS, sulfosuccinimidyl 6-[3′(2-pyridyldithio)-propionamido] hexanoate (Sulfo-LC-SPDP), siRNA Delivery system 2. Doxorubicin-loaded: Glycol-CS, 5β-cholanic acid, doxorubicin | Glycol Sulfo-LC-SPDP Cholanic acid | Solubility Stability (disulphide bonds) Hydrophobic core | In vitro + in vivo | Sequential delivery: 1. NPs doxorubicin 2. NPs siRNA | [86] |
| CS, TAT, siRNA | TAT | Cell uptake | In vitro + in vivo | - | [87] |
| CS, poly(histidine-arginine)6 (H6R6) peptide, siRNA | H6R6 | Cell uptake and endosomal escape | In vitro + in vivo | - | [88] |
| CS, antibody, siRNA | IgG antibody | Target M1 macrophages | In vitro + in vivo | - | [89] |
| Trimethyl CS, PEG, mannose, poly-(allylamine hydro- chloride)-citraconic anhydride (PC) | Trimethyl PEG Mannose PC | Solubility Colloidal stability and longer half-life in blood Target macrophages Endosomal escape | In vitro + in vivo | Co-delivery: 2 siRNAs | [90] |
| CS, PEG, folic acid and diethylethylamine (DEAE) | PEG Folic acid DEAE | Linker Target activated macrophages Colloidal stability | In vitro + in vivo | - | [91] |
| Delivery system 1: Trimethyl-CS, 3 siRNAs Delivery system 2: Imidazole-CS, 3 siRNAs | Trimethyl Imidazole | Solubility and enhanced mucoadhesive properties Endosomal escape | In vitro | Co-delivery: 3 siRNAs | [92] |
| CS, carboxymethyl dextran (CMD), doxorubicin, siRNA | CMD | Colloidal stability and longer half-life | In vitro | Co-delivery: siRNA doxorubicin | [93,94] |
| CS, carboxymethyl dextran (CMD), SN38, siRNA | CMD | Colloidal stability and longer half-life | In vitro | Co-delivery: siRNA SN38 | [95] |
| CS, cholesterol, curcumin, siRNA | Cholesterol | Hydrophobic core | In vitro | Co-delivery: siRNA curcumin | [96] |
| CS-lactate, TPP, siRNA | Lactate | Solubility | In vitro | - | [97] |
| Delivery system 1. Without modification: CS, siRNA Delivery system 2. With modification: CS, PEG, siRNA | PEG | Colloidal stability and longer half-life | In vitro | - | [98] |
| LMw O-carboxymethyl-CS, bPEI, human epidermal growth factor receptor 2 (HER-2/neu), siRNA | LMw CS O-carboxymethyl bPEI HER-2/neu | Solubility and colloidal stability Solubility Cell uptake and endosomal escape Targeting ligand | In vitro | - | [99] |
| CS, PEG, siRNA | PEG | Solubility and stability | In vitro | - | [100] |
| CS, urocanic acid, siRNA | urocanic acid | Endosomal escape and siRNA binding | In vitro | - | [101] |
| CS, PEG, PEI, RGD, siRNA | PEG PEI RGD | Solubility and stability Cell uptake and endosomal escape Target αvβ3 integrin receptors | In vitro | - | [102] |
| CS, PEG, HA, PEI, TPP, siRNA | PEG HA PEI | Solubility and stability Stability Cell uptake and endosomal escape | In vitro | - | [103] |
| Trimethyl CS, cysteine, mannose | Trimethyl Cysteine Mannose | Intestinal mucoadhesion Intestinal mucoadhesion Target enterocites and M cells (intestinal absorption) | In vitro | - | [104] |
| CS, PEG, TAT, siRNA | PEG TAT | Linker between CS and TAT Cell uptake | In vitro | - | [105] |
| CS, nonaarginine, siRNA | nonaarginine | Cell uptake | In vitro | - | [106] |
| CS, PEI, CMD | PEI CMD | Endosomal escape Colloidal stability | In vitro | - | [17] |
| Type of Association between Hyaluronic Acid and Cationic Polymer | Cationic Polymer | Components of the Delivery System | Type of Nanoparticle | In vitro/In vivo Study | Combination Therapy | Ref |
|---|---|---|---|---|---|---|
| Electrostatic interaction | PCD | HA, hyperbranched poly(amido amine) (PCD), siRNA | Polyelectrolyte complex | In vitro | - | [141] |
| PEI | HA-cystamine, Octyl-ss-PEI (reducible bond) or Octyl-cc-PEI (non-reducible bond), paclitaxel, siRNA | Polymeric micelle | In vitro + in vivo | Co-delivery: siRNA paclitaxel | [142] | |
| PEI | HA, PLGA-ss-PEI (reducible bond) or PLGA-PEI (non-reducible bond), docetaxel, siRNA | Polymeric micelle | In vitro + in vivo | Co-delivery: siRNA docetaxel | [143] | |
| LPEI | HA-ss-siRNA (reducible bond) or HA-siRNA (non-reducible bond), LPEI | Polyelectrolyte complex | In vitro + in vivo | - | [140] | |
| bPEI | HA, bPEI, polysiRNA | Polyelectrolyte complex | In vitro + in vivo | - | [138] | |
| CS | HA, CS, TPP, siRNA | Nanogel | In vitro | - | [134] | |
| Protamine | HA-taurocholic acid, protamine, siRNA | Polyelectrolyte complex | In vitro + in vivo | - | [144] | |
| Protamine | HA, Protamine, epigallocatechin-3-O-gallate (EGCG), CPP, siRNA | Nanogel | In vitro + in vivo | Co-delivery: siRNA EGCG | [145] | |
| Covalent linking | bPEI | HA-ss-(Octandioic acid-g-bPEI) or HA-(Octandioic acid-g-bPEI), paclitaxel, siRNA | Polymeric micelle | In vitro + in vivo | Co-delivery: siRNA paclitaxel | [146] |
| bPEI | HA-bPEI, HA-PEG, HA-Hexyl fatty acid, Cholesterol-siRNA | Polymeric micelle | In vitro + in vivo | - | [137] | |
| bPEI | Selected system after HA derivatives screening: HA-PEI, HA-PEG, siRNA or cisplatin Other HA-derivatives studied: HA-butylamine, HA-hexylamine, HA-octylamine, HA-stearylamine, HA-1,6 diaminohexane, HA-1,8 diaminooctane, HA-choline, HA-spermine, | Polyelectrolyte complex | In vitro + in vivo | Sequential delivery: 1. NPs siRNA 2. NPs cisplatin | [147,148,149] | |
| PEI | HA-PEI, siRNA | Polyelectrolyte complex | In vitro + in vivo | - | [150] | |
| PEI | HA-PEI, heparin, Ca2+, siRNA | Polyelectrolyte complex | In vitro | - | [151] | |
| Spermine | HA-spermine, siRNA | Polymeric micelle | In vitro | - | [152] | |
| pDMAEMA | HA-pDMAEMA- 2-(2-pyridyldithio) ethylamine (PDA), siRNA | Polyelectrolyte complex | In vitro + in vivo | - | [153] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serrano-Sevilla, I.; Artiga, Á.; Mitchell, S.G.; De Matteis, L.; de la Fuente, J.M. Natural Polysaccharides for siRNA Delivery: Nanocarriers Based on Chitosan, Hyaluronic Acid, and Their Derivatives. Molecules 2019, 24, 2570. https://doi.org/10.3390/molecules24142570
Serrano-Sevilla I, Artiga Á, Mitchell SG, De Matteis L, de la Fuente JM. Natural Polysaccharides for siRNA Delivery: Nanocarriers Based on Chitosan, Hyaluronic Acid, and Their Derivatives. Molecules. 2019; 24(14):2570. https://doi.org/10.3390/molecules24142570
Chicago/Turabian StyleSerrano-Sevilla, Inés, Álvaro Artiga, Scott G. Mitchell, Laura De Matteis, and Jesús M. de la Fuente. 2019. "Natural Polysaccharides for siRNA Delivery: Nanocarriers Based on Chitosan, Hyaluronic Acid, and Their Derivatives" Molecules 24, no. 14: 2570. https://doi.org/10.3390/molecules24142570
APA StyleSerrano-Sevilla, I., Artiga, Á., Mitchell, S. G., De Matteis, L., & de la Fuente, J. M. (2019). Natural Polysaccharides for siRNA Delivery: Nanocarriers Based on Chitosan, Hyaluronic Acid, and Their Derivatives. Molecules, 24(14), 2570. https://doi.org/10.3390/molecules24142570