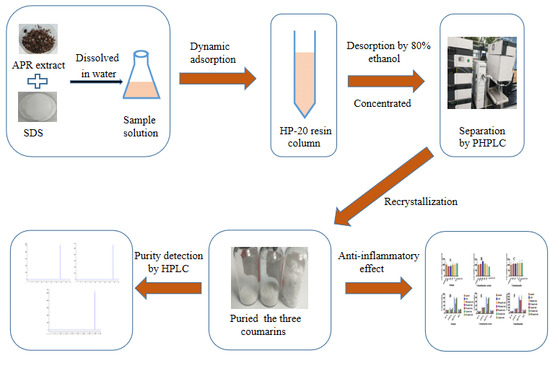
Separation and Enrichment of Three Coumarins from Angelicae Pubescentis Radix by Macroporous Resin with Preparative HPLC and Evaluation of Their Anti-Inflammatory Activity
Abstract
:1. Introduction
2. Result and Discussion
2.1. Optimization of Resin
2.2. Adsorption Dynamics
2.3. Adsorption Isotherms and Thermodynamics
2.4. Dynamic Adsorption and Desorption
2.5. Enrichment of Three Coumarins
2.6. Separation of Three Coumarins by PHPLC
2.7. Anti-Inflammation Activity of the Three Coumarins
3. Materials and Methods
3.1. Samples and Chemicals
3.2. Adsorbents
3.3. Preparation of APR Extract
3.4. HPLC Analysis of Three Coumarin Compounds
3.5. Static Adsorption and Desorption Experiments
3.5.1. Optimization of Resin
3.5.2. Adsorption Dynamics
3.5.3. Adsorption Isotherms
3.5.4. Adsorption Thermodynamics
3.6. Dynamics Adsorption and Desorption Experiment
3.7. Enrichment of Three Coumarins by HP-20 Resin
3.8. Separation of Three Coumarins by PHPLC
3.9. The Anti-Inflammatory Effect of the Three Coumarins on RAW264.7 Macrophages
3.9.1. RAW264.7 Cell Viability Assay
3.9.2. Effects of These Three Coumarins on the Release of Inflammatory Factors by LPS Induced in RAW264.7 Cells
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Editorial Committee of the Pharmacopoeia of the People’s Republic of China. Pharmacopoeia of the People’s Republic of China; Chemical Technology Press: Shanghai, China, 2015; pp. 263–264.
- Liu, J.H.; Xu, S.X.; Yao, X.S. Chemical Constituents and Pharmacology Research Review of Angelicae Pubescentis Radix. J. Shenyang Coll. Pharm. 1994, 11, 143–150. [Google Scholar]
- Chang, Y.X.; Zhu, Z.W.; Li, J.; Zhang, Q.H.; Qin, X.W. Simultaneous Determination of the Seven Major Constituents for Quality Control of Radix Angelicae Pubescentis by HPLC Coupled with Chemometrics Method. J. Inn. Mong. Univ. 2011, 3. [Google Scholar]
- Li, R.L.; Zhao, C.; Yao, M.N.; Song, Y.; Wu, Y.; Wen, A.D. Analgesic effect of coumarins from Radix angelicae pubescentis is mediated by inflammatory factors and TRPV1 in a spared nerve injury model of neuropathic pain. J. Ethnopharmacol. 2017, 195, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.Y.; Yao, L.; Du, Y.H.; Xie, J.B.; Huang, J.L.; Yin, Z.Q. Anthelmintic activity of the crude extracts, fractions, and osthole from Radix angelicae pubescentis against Dactylogyrus intermedius goldfish (Carassius auratus) in vivo. Parasitol. Res. 2011, 108, 195–200. [Google Scholar] [CrossRef]
- Liang, H.J.; Suk, F.M.; Wang, C.K.; Hung, L.F.; Liu, D.Z.; Chen, N.Q.; Chen, Y.C.; Chang, C.C.; Liang, Y.C. Osthole, a potential antidiabetic agent, alleviates hyperglycemia in db/db mice. Chem.-Biol. Interact. 2009, 181, 309–315. [Google Scholar] [CrossRef]
- Lim, H.J.; Lee, J.H.; Choi, J.S.; Lee, S.K.; Kim, Y.S.; Kim, H.P. Inhibition of airway inflammation by the roots of Angelica decursiva and its constituent columbianadin. J. Ethnopharmacol. 2014, 155, 1353–1361. [Google Scholar] [CrossRef]
- Chen, Y.F.; Tsai, H.Y.; Wu, T.S. Anti-inflammatory and analgesic activities from roots of Angelica pubescens. Planta Med. 1995, 61, 2–8. [Google Scholar] [CrossRef]
- Liu, L.; Mao, J.; Wang, Q.F.; Zhang, Z.W.; Wu, G.Z.; Tang, Q.Z.; Zhao, B.; Li, L.H.; Li, Q.L. In vitro anticancer activities of osthole against renal cell carcinoma cells. Biomed. Pharmacother. 2017, 94, 1020–1027. [Google Scholar] [CrossRef]
- Cheng, G.J.S.; Li, G.K.; Xiao, X.H. Microwave-assisted extraction coupled with counter-current chromatography and preparative liquid chromatography for the preparation of six furocoumarins from Angelica Pubescentis Radix. Sep. Purif. Technol. 2015, 141, 143–149. [Google Scholar] [CrossRef]
- Yan, Z.H.; Huang, X.Z.; Dunzhu, C.R.; Liu, Z.Y.; Xiong, Y.K.; Xie, Y.H.; Zhu, G.H. Separation and purification of coumarins from Heracleum millefolium by high-speed counter-current chromatography. Chin. Tradit. Herb. Drugs 2015, 46, 3023–3027. [Google Scholar]
- Zhang, C.Y.; Zhang, B.G.; Yang, X.W. Studies on the Chemical constituents of the Root of Angelica pubescens f.biserrata. Pharm. J. Chin. People’s Lib. Army 2007, 4, 241–245. [Google Scholar]
- Han, F.; Guo, Y.P.; Gu, H.Y.; Li, F.L.; Hu, B.Z.; Yang, L. Application of alkyl polyglycoside surfactant in ultrasonic-assisted extraction followed by macroporous resin enrichment for the separation of vitexin-2′′-O-rhamnoside and vitexin from Crataegus pinnatifida leaves. J. Chromatogr. B 2016, 1012–1013, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Leyton, A.; Vergara-Salinas, J.R.; Pérez-Correa, J.R.; Lienqueo, M.E. Purification of phlorotannins from macrocystis pyrifera using macroporous resins. Food Chem. 2017, 237, 312–319. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yoon, M.; Yang, H.; Jo, J.; Han, D.; Jeon, Y.; Cho, S. Enrichment and purification of marine polyphenol phlorotannins using macroporous adsorption resins. Food Chem. 2014, 162, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.L.; Gao, X.; Fu, Q.; Guo, P.Q.; Xu, X.Y.; Zhang, T.; Ge, Y.H.; Zhang, B.L.; Wang, M.C.; Zeng, A.G.; et al. Enrichment of total steroidal saponins from the extracts of Trillium tschonoskii Maxim by macroporous resin and the simultaneous determination of eight steroidal saponins in the final product by HPLC. J. Sep. Sci. 2017, 40, 1115–1124. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Sun, X.Y.; Li, X.W.; Yang, T.; Qing, L.W. Enrichment and separation of quercetin-3-O-β-d-glucuronide from lotus leaves (nelumbo nucifera gaertn.) and evaluation of its anti-inflammatory effect. J. Chromatogr. B 2017, 1040, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Li, H.J.; Liu, Y.; Yi, Y.T.; Miao, Q.; Liu, S.J.; Zhao, F.; Cong, W.; Wang, C.H.; Xia, C.H. Purification of quercetin-3-0-sophoroside and isoquercitrin from Poacynum hendersonii leaves using macroporous resins followed by Sephadex LH-20 column chromatography. J. Chromatogr. B 2017, 1048, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Li, H.J.; Hou, G.G.; Liu, Y.Y.; Zhao, F.; Cong, W.; Wang, C.H. Preparative separation of phloridzin from apple leaves using macroporous resins followed by preparative high-performance liquid chromatography. J. Sep. Sci. 2018, 41, 3818–3924. [Google Scholar] [CrossRef]
- Namasivayam, C.; Ranganathan, K. Waste Fe (III)/Cr (III) hydroxide as adsorbent for the removal of Cr (VI) from aqueous solution and chromium plating industry waste water. Environ. Pollut. 1993, 82, 255–261. [Google Scholar] [CrossRef]
- Veli, S.; Alyüz, B. Adsorption of copper and zinc from aqueous solutions by using natural clay. J. Hazard. Mater. 2007, 149, 226–233. [Google Scholar] [CrossRef]
- Sun, X.C.; Zhang, C.M.; Li, J.N.; Feng, J.L.; Zhou, H.G.; Fu, S.; Chen, W.Q. Chemical constituents from Peucedanum decursivum. Chin. Tradit. Herb. Drugs 2013, 44, 2044–2047. [Google Scholar]
- Wang, L.; Sun, L.; Liu, H.Y.; Meng, F.H. Chemical constituents of Gelsemium elegans. Chin. Tradit. Herb. Drugs 2017, 48, 2028–2032. [Google Scholar]
- Deng, G.G.; Yang, X.W.; Zhang, Y.B.; Xu, W.; Wei, W.; Li, Z.G. Chemical constituents from lipophilic parts in roots of Angelica dahurica cv Yubaizhi. Chinaj. Chin. Mater. Med. 2017, 42, 2102–2109. [Google Scholar]
- Chen, Q.Q. Clinical Research of Duhuo Jisheng Decoction Combined with Meloxicam on Treating Rheumatoid Arthritis. J. Liaoning Univ. Tradit. Chin. Med. 2017, 19, 205–208. [Google Scholar]
- Zhang, K.C. Clinical study on 75 cases of Rheumatoid Arthritis treated by Duhuo Jisheng Decoction. Asia-Pac. Tradit. Med. 2015, 11, 137–138. [Google Scholar]
- Wang, W. The clinical efficacy of Duhuo Jisheng Decoction combined with Acupuncture in treating Rheumatoid Arthritis and its effect on inflammatory factors. Jiangxi Med. J. 2018, 53, 48–51. [Google Scholar]
Sample Availability: Samples of the compounds columbianetin acetate, osthole and columbianadin are available from the authors. |







| Resins | Structure | Polarity | Particle Size (mm) | Pore Diameter (nm) | Surface Area (m2/g) |
|---|---|---|---|---|---|
| D101 | polystyrene | Non-polar | 0.30–1.25 | 9–15 | 480–550 |
| AB-8 | polystyrene | Weak-polar | 0.30–1.25 | 13–14 | 480–520 |
| DA-201 | polystyrene | Polar | 0.30–1.25 | 10–13 | ≧200 |
| HP-20 | polystyrene | Non-polar | 0.30–1.25 | 29–30 | 550–600 |
| GDX-201 | polydivinylbenzene | Non-polar | 0.30–1.25 | — | 510 |
| Kinetics Model | Parameters | Columbianetin Acetate | Osthole | Columbianadin |
|---|---|---|---|---|
| Pseudo-first-order | Qe (mg·g−1) | 1.2748 | 5.5582 | 1.7411 |
| K1 (min−1) | 0.0880 | 0.1022 | 0.0890 | |
| R2 | 0.8424 | 0.9066 | 0.9176 | |
| Pseudo-second-order | Qe (mg·g−1) | 1.3508 | 5.8824 | 1.8512 |
| K2 (g·mg−1·min−1) | 0.1210 | 0.0322 | 0.0851 | |
| R2 | 0.9971 | 0.9927 | 0.9942 | |
| Intra-particle diffusion (10–360 min) | I (mg·g−1) | 0.9619 | 4.4638 | 1.3361 |
| Ki (mg·g−1·min−0.5) | 0.0233 | 0.0813 | 0.0295 | |
| R2 | 0.6952 | 0.6104 | 0.5843 | |
| Intra-particle diffusion (10–40 min) | I (mg·g−1) | 0.5348 | 2.4562 | 0.6456 |
| Ki (mg·g−1·min−0.5) | 0.1031 | 0.4633 | 0.1606 | |
| R2 | 0.9775 | 0.9538 | 0.9700 | |
| Intra-particle diffusion (50–120 min) | I (mg·g−1) | 1.0605 | 5.0046 | 1.5383 |
| Ki (mg·g−1·min−0.5) | 0.0213 | 0.0563 | 0.0192 | |
| R2 | 0.9885 | 0.9465 | 0.9796 | |
| Intra-particle diffusion (180–360 min) | I (mg·g−1) | 1.2795 | 5.6076 | 1.7495 |
| Ki (mg·g−1·min−0.5) | 0.0026 | 0.0055 | 0.0022 | |
| R2 | 0.9580 | 0.9402 | 0.8516 |
| Compound | Temperature (K) | Langmuir Equation | Freundlich Equation | DR Equation | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| R2 | KL | Q0 (mg/g) | RL | R2 | KF | 1/n | Q0 (mg/g) | R2 | KDR | E | ||
| Columbianetin acetate | 298 | 0.9929 | 318.90 | 3.14 | 0.103 | 0.9927 | 8.09 | 0.2804 | 4.20 | 0.9946 | 4.69 × 10−9 | 10.33 × 103 |
| 308 | 0.9936 | 352.14 | 3.38 | 0.094 | 0.9962 | 9.73 | 0.2977 | 4.73 | 0.9987 | 4.51 × 10−9 | 10.53 × 103 | |
| 318 | 0.9917 | 391.10 | 3.50 | 0.086 | 0.9968 | 10.58 | 0.3016 | 4.98 | 0.9954 | 4.16 × 10−9 | 10.97 × 103 | |
| Osthole | 298 | 0.9898 | 125.56 | 14.75 | 0.065 | 0.9975 | 28.29 | 0.2748 | 17.14 | 0.9847 | 5.62 × 10−9 | 9.43 × 103 |
| 308 | 0.9901 | 149.77 | 15.53 | 0.055 | 0.9997 | 32.27 | 0.2848 | 18.59 | 0.9875 | 5.19 × 10−9 | 9.81 × 103 | |
| 318 | 0.9872 | 179.43 | 15.92 | 0.046 | 0.9983 | 34.47 | 0.2830 | 19.27 | 0.9790 | 4.58 × 10−9 | 10.45 × 103 | |
| Columbianadin | 298 | 0.9911 | 386.73 | 4.97 | 0.066 | 0.9923 | 15.17 | 0.3057 | 7.09 | 0.9939 | 4.81 × 10−9 | 10.20 × 103 |
| 308 | 0.9933 | 415.00 | 5.02 | 0.062 | 0.9937 | 15.56 | 0.3047 | 7.23 | 0.9987 | 4.45 × 10−9 | 10.60 × 103 | |
| 318 | 0.9911 | 492.82 | 5.20 | 0.053 | 0.9948 | 17.42 | 0.3103 | 7.71 | 0.9960 | 4.08 × 10−9 | 11.08 × 103 | |
| Compound | Temperature (K) | ∆G0 (kJ/mol) | ∆H0 (kJ/mol) | ∆S0 (J/mol) |
|---|---|---|---|---|
| Columbianetin acetate | 298 | −29.27 | 8.03 | 121.97 |
| 308 | −29.52 | |||
| 318 | −29.79 | |||
| Osthole | 298 | −25.60 | 14.06 | 133.05 |
| 308 | −26.03 | |||
| 318 | −26.48 | |||
| Columbianadin | 298 | −31.07 | 9.50 | 129.45 |
| 308 | −31.26 | |||
| 318 | −31.71 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Zhu, R.; Li, J.; Yang, X.; He, J.; Wang, H.; Chang, Y. Separation and Enrichment of Three Coumarins from Angelicae Pubescentis Radix by Macroporous Resin with Preparative HPLC and Evaluation of Their Anti-Inflammatory Activity. Molecules 2019, 24, 2664. https://doi.org/10.3390/molecules24142664
Yang Y, Zhu R, Li J, Yang X, He J, Wang H, Chang Y. Separation and Enrichment of Three Coumarins from Angelicae Pubescentis Radix by Macroporous Resin with Preparative HPLC and Evaluation of Their Anti-Inflammatory Activity. Molecules. 2019; 24(14):2664. https://doi.org/10.3390/molecules24142664
Chicago/Turabian StyleYang, Yuqiao, Ruichao Zhu, Jin Li, Xuejing Yang, Jun He, Hui Wang, and Yanxu Chang. 2019. "Separation and Enrichment of Three Coumarins from Angelicae Pubescentis Radix by Macroporous Resin with Preparative HPLC and Evaluation of Their Anti-Inflammatory Activity" Molecules 24, no. 14: 2664. https://doi.org/10.3390/molecules24142664
APA StyleYang, Y., Zhu, R., Li, J., Yang, X., He, J., Wang, H., & Chang, Y. (2019). Separation and Enrichment of Three Coumarins from Angelicae Pubescentis Radix by Macroporous Resin with Preparative HPLC and Evaluation of Their Anti-Inflammatory Activity. Molecules, 24(14), 2664. https://doi.org/10.3390/molecules24142664